Selective 6π-Electrocyclization of N-Vinyl-α,β-Unsaturated Nitrones to Prepare Polysubstituted Pyridine Derivatives
Li-Yao Ding
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), Collaborative Innovation Center for Guangxi Ethnic Medicine, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, 15 Yu Cai Road, Guilin, Guangxi, 541004 China
These authors contributed equally.
Search for more papers by this authorYan-Jiao Lu
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), Collaborative Innovation Center for Guangxi Ethnic Medicine, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, 15 Yu Cai Road, Guilin, Guangxi, 541004 China
These authors contributed equally.
Search for more papers by this authorJin-Hong Pang
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), Collaborative Innovation Center for Guangxi Ethnic Medicine, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, 15 Yu Cai Road, Guilin, Guangxi, 541004 China
Search for more papers by this authorHai-Fang Lin
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), Collaborative Innovation Center for Guangxi Ethnic Medicine, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, 15 Yu Cai Road, Guilin, Guangxi, 541004 China
Search for more papers by this authorCorresponding Author
Chun-Hua Chen
Key Laboratory of Chemistry and Engineering of Forest Products, State Ethnic Affairs Commision, Guangxi Key Laboratory of Chemistry and Engineering of Forest Products, Guangxi Collaborative Innovation Center for Chemistry and Engineering of Forest Products, School of Chemistry and Chemical Engineering, Guangxi Minzu University, Nanning, Guangxi, 530006 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Hong-Yan Bi
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), Collaborative Innovation Center for Guangxi Ethnic Medicine, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, 15 Yu Cai Road, Guilin, Guangxi, 541004 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Dong-Liang Mo
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), Collaborative Innovation Center for Guangxi Ethnic Medicine, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, 15 Yu Cai Road, Guilin, Guangxi, 541004 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorLi-Yao Ding
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), Collaborative Innovation Center for Guangxi Ethnic Medicine, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, 15 Yu Cai Road, Guilin, Guangxi, 541004 China
These authors contributed equally.
Search for more papers by this authorYan-Jiao Lu
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), Collaborative Innovation Center for Guangxi Ethnic Medicine, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, 15 Yu Cai Road, Guilin, Guangxi, 541004 China
These authors contributed equally.
Search for more papers by this authorJin-Hong Pang
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), Collaborative Innovation Center for Guangxi Ethnic Medicine, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, 15 Yu Cai Road, Guilin, Guangxi, 541004 China
Search for more papers by this authorHai-Fang Lin
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), Collaborative Innovation Center for Guangxi Ethnic Medicine, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, 15 Yu Cai Road, Guilin, Guangxi, 541004 China
Search for more papers by this authorCorresponding Author
Chun-Hua Chen
Key Laboratory of Chemistry and Engineering of Forest Products, State Ethnic Affairs Commision, Guangxi Key Laboratory of Chemistry and Engineering of Forest Products, Guangxi Collaborative Innovation Center for Chemistry and Engineering of Forest Products, School of Chemistry and Chemical Engineering, Guangxi Minzu University, Nanning, Guangxi, 530006 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Hong-Yan Bi
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), Collaborative Innovation Center for Guangxi Ethnic Medicine, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, 15 Yu Cai Road, Guilin, Guangxi, 541004 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Dong-Liang Mo
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), Collaborative Innovation Center for Guangxi Ethnic Medicine, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, 15 Yu Cai Road, Guilin, Guangxi, 541004 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
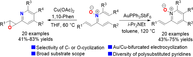
Herein, we have developed a facile method for the synthesis of various polysubstituted pyridine derivatives through selective 6π-electrocyclization of N-vinyl-α,β-unsaturated nitrones. It was found that gold catalysts promoted carbon-6π-electrocyclization of N-vinyl-α,β-unsaturated nitrones to afford 6-alkenyl pyridine N-oxides in 43%—75% yields, whereas copper catalysts facilitated oxygen-6π-electrocyclization to give 6-epoxy pyridines in 41%—83% yields. The present method features broad substrate scope, good functional group tolerance, high cyclization selectivity, and diversity of polysubstituted pyridine scaffolds.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202401287-sup-0001-supinfo.pdfPDF document, 5.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Schuppe, A. W.; Liu, Y.; Gonzalez-Hurtado, E.; Zhao, Y.; Jiang, X.; Ibarraran, S.; Huang, D.; Wang, X.; Lee, J.; Loria, J. P.; Dixit, V. D.; Li, X.; Newhouse, T. R. Unified total synthesis of the limonoid alkaloids: Strategies for the de novo synthesis of highly substituted pyridine scaffolds. Chem 2022, 8, 2856–2887; (b) Liu, W.-S.; Yang, B.; Wang, R.-R.; Li, W.-Y.; Ma, Y.-C.; Zhou, L.; Du, S.; Ma, Y.; Wang, R.-L. Design, synthesis and biological evaluation of pyridine derivatives as selective SHP2 inhibitors. Bioorg. Chem. 2020, 100, 103875; (c) Desimoni, G.; Faita, G.; Quadrelli, P. Pyridine-2,6-bis-(oxazolines), Helpful Ligands for Asymmetric Catalysts. Chem. Rev. 2003, 103, 3119–3154.
- 2(a) Allais, C.; Grassot, J.-M.; Rodriguez, J.; Constrantieux, T. Metal- Free Multicomponent Syntheses of Pyridines. Chem. Rev. 2014, 114, 10829–10868; (b) Reza, A. I.; Iwai, K.; Nishiwaki, N. Recent Advances in Synthesis of Multiply Arylated/Alkylated Pyridines. Chem. Rec. 2022, 22, e202200099; (c) Hill, M. D. Recent Strategies for the Synthesis of Pyridine Derivatives. Chem. Eur. J. 2010, 16, 12052–12062.
- 3(a) Hu, Z.; Zhang, M.; Zhou, Q.; Xu, X.; Tang, B. Domino synthesis of fully substituted pyridines by silver-catalyzed chemoselective hetero-dimerization of isocyanides. Org. Chem. Front. 2020, 7, 507–512; (b) Tan, J.-F.; Bormann, C. T.; Perrin, F. G.; Chadwick, F. M.; Severin, K.; Cramer, N. Divergent Synthesis of Densely Substituted Arenes and Pyridines via Cyclotrimerization Reactions of Alkynyl Triazenes. J. Am. Chem. Soc. 2019, 141, 10372–10383.
- 4(a) Duan, Y.; Wang, Y.; Li, D. A Facile Approach for Synthesis of Benzofuro[2,3-c]pyridines via Intramolecular Cascade Annulations. Chin. J. Chem. 2014, 32, 1103–1106; (b) Nie, B.; Wu, W.; Ren, Q.; Wang, Z.; Zhang, J.; Zhang, Y.; Jiang, H. Access to Cycloalkeno[c]- Fused Pyridines via Pd-Catalyzed C(sp2)–H Activation and Cyclization of N-Acetyl Hydrazones of Acylcycloalkenes with Vinyl Azides. Org. Lett. 2020, 22, 7786–7790; (c) Hu, Y.; Shi, W.; Yan, Z.; Liao, J.; Liu, M.; Xu, J.; Wang, W.; Wu, Y.; Zhang, C.; Guo, H. Base-Catalyzed Sequential 1,4-Addition/Intramolecular Cyclization/Aromatization Reaction: Synthesis of Benzofuro[3,2-b]pyridines. Org. Lett. 2021, 23, 6780–6783; (d) Bao, L.; Liu, Y.; Peng, J.; Wang, Y.; Dong, J.; Xu, X. Chemoselective Trimerization of Isocyanides: De Novo Synthesis of 2-Indole-Substituted Quinolines and Pyridines. Org. Lett. 2022, 24, 105–109.
- 5(a) Guo, H.; Qiu, S.; Xu, P. One-carbon ring expansion of indoles and pyrroles: a straightforward access to 3-fluorinated quinolines and pyridines. Angew. Chem. Int. Ed. 2024, 63, e202317104; (b) Joynson, B. W.; Cumming, G. R.; Ball, L. T. Photochemically mediated ring expansion of indoles and pyrroles with chlorodiazirines: synthetic methodology and thermal hazard assessment. Angew. Chem. Int. Ed. 2023, 62, e202305081; (c) Reisenbauer, J. C.; Green, O.; Franchino, A.; Finkelstein, P.; Morandi, B. Late-stage diversification of indole skeletons through nitrogen atom insertion. Science 2022, 377, 1104–1109.
- 6(a) Chang, L.; Lai, J.; Yuan, G. One-Pot Synthesis of Hantzsch Pyridines via NH4I Promoted Condensation of 1,3-Dicarbonyl Compounds with DMSO and NH4OAc. Chin. J. Chem. 2016, 34, 887–894; (b) Cai, W.; Wang, S.; Jalani, H. B.; Wu, J.; Lu, H.; Li, G. Oxidative Cascade Reaction of N-Aryl-3-alkylideneazetidines and Carboxylic Acids: Access to Fused Pyridines. Org. Lett. 2018, 20, 3833–3837; (c) Teng, Q.-H.; Peng, X.-J.; Mo, Z.-Y.; Xu, Y.-L.; Tang, H.-T.; Wang, H.-S.; Sun, H.-B.; Pan, Y.-M. Transition-metal-free C–N and C–C formation: synthesis of benzo[4,5]imidazo[1,2-a]pyridines and 2-pyridones from ynones. Green Chem. 2018, 20, 2007–2012; (d) Zhang, Y.; Luo, H.; Lu, Q.; An, Q.; Li, Y.; Li, S.; Tang, Z.; Li, B. Access to pyridines via cascade nucleophilic addition reaction of 1,2,3-triazines with activated ketones or acetonitriles. Chin. Chem. Lett. 2021, 32, 393–396; (e) Cai, C.-Y.; Chen, S.-J.; Merchant, R. R.; Kanda, Y.; Qin, T. C3 Selective Hydroxylation of Pyridines via Photochemical Valence Isomerization of Pyridine N-Oxides. J. Am. Chem. Soc. 2024, 146, 24257–24264.
- 7Recent reviews for 6π-azaelectrocyclization, see: (a) Vargas, D. F.; Larghi, E. L.; Kaufman, T. S. The 6π-Azaelectrocyclization of Azatrienes. Synthetic Applications in Natural Products, Bioactive Heterocycles, and Related Fields. Nat. Prod. Rep. 2019, 36, 354–401; (b) Katsunori, T.; Shigeo, K.; Koichi, F. Discovery and application of 6π-Azaelectrocyclization to natural product synthesis and synthetic biology. Sci. China Chem. 2012, 55, 19–30; (c) Sun, D.; Confair, D. N.; Ellman, J. A. Rhodium-Catalyzed C−H Alkenylation/Electrocyclization Cascade Provides Dihydropyridines That Serve as Versatile Intermediates to Diverse Nitrogen Heterocycles. Acc. Chem. Res. 2021, 54, 1766–1778; (d) Huang, Z.; Liu, W.; Zhang, W.-X. Overview of 6π 1,5-Electrocyclization over the Past 40 Years. Chin. J. Chem. 2023, 41, 725–740.
- 8(a) Liu, X.; Zhang, N.; Yang, J.; Liang, Y.; Zhang, R.; Dong, D. Hydrogen Bond-Assisted 6π-Azaelectrocyclization of Penta-2,4-dienamides: Synthesis of Dihydropyridin-2(3H)-ones. J. Org. Chem. 2013, 78, 3323–3328; (b) Wei, H.; Li, Y.; Xiao, K.; Cheng, B.; Wang, H.; Hu, L.; Zhai, H. Synthesis of Polysubstituted Pyridines via a One-Pot Metal- Free Strategy. Org. Lett. 2015, 17, 5974–5977; (c) Mora-Radó, H.; Birly, L.; Czechtizky, W.; Méndez, M; Harrity, J. P. A. An Alkyne Diboration/6π-Electrocyclization Strategy for the Synthesis of Pyridine Boronic Acid Derivatives. Angew. Chem. Int. Ed. 2016, 55, 5834–5836.
- 9(a) Saini, K. M.; Saunthwal, R. K.; Kumar, A.; Verma, A. K. Tandem 6π-Azatriene Electrocyclization of Fused Aminocyclopentenones: Synthesis of Functionalized Pyrrolo- and Indolo-quinoxalines. Org. Lett. 2021, 23, 7586–7591; (b) Shi, L.; Pan, L.; Li, Y.; Liu, Q. Copper(II)- Catalyzed Aerobic Oxidative Desulfitative 6π Electrocyclization: Efficient Synthesis of Diverse 4-Aminoquinolines. Adv. Synth. Catal. 2017, 359, 2457–2470.
- 10(a) Zhu, X.-Q.; Wang, Z.-S.; Hou, B.-S.; Zhang, H.-W.; Deng, C.; Ye, L.-W. Zinc-Catalyzed Asymmetric Formal [4+3] Annulation of Isoxazoles with Enynol Ethers by 6π Electrocyclization: Stereoselective Access to 2H-Azepines. Angew. Chem. Int. Ed. 2020, 59, 1666–1673; (b) Das, A.; Volla, C. M. R.; Atodiresei, I.; Bettray, W.; Rueping, M. Asymmetric Ion Pair Catalysis of 6π Electrocyclizations: Brønsted Acid Catalyzed Enantioselective Synthesis of Optically Active 1,4-Dihydropyridazines. Angew. Chem. Int. Ed. 2013, 52, 8008–8011.
- 11(a) Zhao, Z.; Wei, H.; Xiao, K.; Cheng, B.; Zhai, H.; Li, Y. Facile Synthesis of Pyridines from Propargyl Amines: Concise Total Synthesis of Suaveoline Alkaloids. Angew. Chem. Int. Ed. 2019, 58, 1148–1152; (b) Cheng, X.; Waters, S. P. Pyridone Annulation via Tandem Curtius Rearrangement/6π-Electrocyclization: Total Synthesis of (-)-Lyconadin C. Org. Lett. 2013, 15, 4226–4229; (c) Li, M.-F.; Shi, S.-Q.; Xu, T.; Zhang, Q.; Hoa, W.-J.; Wang, S.-L.; Wang, J.; Tu, S.-J.; Jiang, B. Stereoselective construction of azepine-containing bridged scaffolds via organocatalytic bicyclization of yne-allenone esters with nitrones. Chin. Chem. Lett. 2023, 34, 107751.
- 12(a) Bilodeau, D. A.; Margison, K. D.; Serhan, M.; Pezacki, J. P. Bioorthogonal Reactions Utilizing Nitrones as Versatile Dipoles in Cycloaddition Reactions. Chem. Rev. 2021, 121, 6699–6717; (b) Shi, W.-M.; Ma, X.-P.; Su, G.-F.; Mo, D.-L. New developments of ketonitrones in organic synthesis. Org. Chem. Front. 2016, 3, 116–130; (c) Zou, N.; Qin, X.-T.; Wang, Z.-X.; Shi, W.-M.; Mo, D.-L. Advances on the Synthesis and Application of α,β-Unsaturated Nitrones. Chin. J. Org. Chem. 2021, 41, 4535–4553; (d) Li, T.-Z.; Liu, S.-J.; Sun, Y.-W.; Deng, S.; Tan, W.; Jiao, Y.; Zhang, Y.-C.; Shi, F. Regio- and Enantioselective [3+3] Cycloaddition of Nitrones with 2-Indolylmethanols Enabled by Cooperative Organocatalysis. Angew. Chem. Int. Ed. 2021, 60, 2355–2363; (e) Wan, M.; Yao, M.; Gong, J.-Y.; Yang, P.; Liu, H.; Li, A. Synthesis of the tetracyclic core of chlorospermines. Chin. Chem. Lett. 2015, 26, 272–276.
- 13(a) Reidl, T. W.; Son, J.; Wink, D. J.; Anderson, L. L. Facile Synthesis of Azetidine Nitrones and Diastereoselective Conversion into Densely Substituted Azetidines. Angew. Chem. Int. Ed. 2017, 56, 11579–11583; (b) Lu, Y.-J.; Lu, F.-L.; Zhang, J.-Q.; Chen, C.-H.; Liang, C.; Ma, X.-P.; Mo, D.-L. Iron(III)/Quinoxaline-Derived N,N-Ligand Catalyzed Oxygen Transfer Reaction of N-Vinyl Nitrones through Selective 4π-Electrocyclization and N-O Bond Cleavage. Org. Chem. Front. 2024, 11, 2277–2282; (c) Zhang, J.-Q.; Qiu, P.-W.; Liang, C.; Mo, D.-L. Synthesis of Azetidine Nitrones and Exomethylene Oxazolines through a Copper(I)-Catalyzed 2,3-Rearrangement and 4π-Electrocyclization Cascade Strategy. Org. Lett. 2022, 24, 7801–7805; (d) Wu, Y.-Z.; Leng, Y.; Chen, Y.-X.; Huang, S.-Q.; Zou, N.; Chen, C.-H.; Mo, D.-L. Yb(OTf)3-Catalyzed Asymmetric [3+3] Cycloaddition of N-Vinyl Nitrones with Activated Cyclopropanes to Prepare 1,2-Oxazines. Chin. J. Chem. 2025, 43, 417–422.
- 14 Nakamura, I.; Zhang, D.; Terada, M. Copper-Catalyzed Tandem [2,3]-Rearrangement and 6π-3-Azatriene Electrocyclization in (E)-O-Propargylic α,β-Unsaturated Oximes. J. Am. Chem. Soc. 2010, 132, 7884–7886.
- 15 Son, J.; Kim, K. H.; Mo, D.-L.; Wink, D. J.; Anderson, L. L. Single-Step Modular Synthesis of Unsaturated Morpholine N-Oxides and Their Cycloaddition Reactions. Angew. Chem. Int. Ed. 2017, 56, 3059–3063.
- 16(a) Ma, X.-P.; Nong, C.-M.; Liang, Y.-F.; Xu, P.-P.; Guo, X.-Y.; Liang, C.; Pan, C.-X.; Su, G.-F.; Mo, D.-L. An Yb(OTf)3 and Visible Light Relay Catalyzed [3+2] Cycloaddition/[3,3]-Rearrangement/[4+2] Cycloaddition in One Pot to Prepare Oxazonine-Fused Endoperoxides. Green Chem. 2020, 22, 3827–3834;
(b) Zou, N.; Jiao, J.-W.; Feng, Y.; Pan, C.-X.; Liang, C.; Su, G.-F.; Mo, D.-L. Iron(III)/Copper(II)-Cocatalyzed Cycloaddition/[3,3]-Rearrangement/N-O Bond Cleavage to Prepare Polysubstituted Pyrrolizines from N-Vinyl-α,β-Unsaturated Nitrones and Activated Alkynes. Org. Lett. 2019, 21, 481–485;
(c) Luo, Y.; Lu, Y.-J.; Pan, M.-M.; Liang, Y.-F.; Shi, W.-M.; Chen, C.-H.; Liang, C.; Su, G.-F.; Mo, D.-L. Rapidly diastereoselective assembly of ten-membered N-heterocycles between two 1,3-dipoles and their diversity to access fused N-heterocycles. Chin. Chem. Lett. 2024, DOI: https://doi.org/10.1016/j.cclet.2024.110207.
10.1016/j.cclet.2024.110207 Google Scholar
- 17(a) Chen, C.-H.; Wu, Q.-Y.; Wei, C.; Liang, C.; Su, G.-F.; Mo, D.-L. Iron(III)-catalyzed selective N–O bond cleavage to prepare tetrasubstituted pyridines and 3,5-disubstituted isoxazolines from N-vinyl- α,β-unsaturated ketonitrones. Green Chem. 2018, 20, 2722–2729; (b) Zou, N.; Lan, J.-X.; Yan, G.-G.; Liang, C.; Su, G.-F.; Mo, D.-L. Nickel(II)- Catalyzed Oxygen Transfer Reaction of N-Vinyl Nitrones to Prepare 2-(Pyridin-2-yl)ethanols, Org. Lett. 2020, 22, 8446–8450; (c) Zou, N.; Liu, Z.-W.; Yan, G.-G.; Wang, Y.-C.; Liang, C.; Mo, D.-L. DBU-Promoted 6π-Azaelectrocyclization and Hydrogen-Migration to Prepare 6-Alkyl Pyridine N-Oxides from N-Vinyl-α,β-Unsaturated Nitrones. Adv. Synth. Catal. 2022, 364, 1671–1676.
- 18CCDCs: 2133594 (2b) and 2410557 (3s) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.