Improving Pure Organic Room-Temperature Phosphorescence by Substituent Effect of Thianthrene
Zhe Feng
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorZhiqiang Yang
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorShuaiqiang Zhao
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorJunjie Qian
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorShi-Tong Zhang
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorCorresponding Author
Haichao Liu
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Bing Yang
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
E-mail: [email protected]; [email protected]Search for more papers by this authorZhe Feng
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorZhiqiang Yang
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorShuaiqiang Zhao
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorJunjie Qian
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorShi-Tong Zhang
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorCorresponding Author
Haichao Liu
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Bing Yang
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
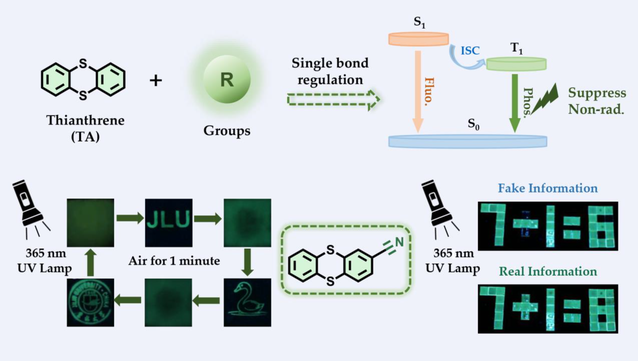
To gain insights into the potential of thianthrene (TA), its substituent effects were systematically studied on the room-temperature phosphorescence (RTP) properties, including the electron-donating and electron-withdrawing substituents at 1- and 2-positions of TA, respectively. Both theoretical and experimental investigations show that the 2-position electron-withdrawing substituents greatly enhance RTP performance than the 1-position substituents, while the situation is exactly the opposite for electron-donating substituents. Compared with the 1-position substitution, the 2-position electron-withdrawing substituents induce the higher RTP radiation rate and lower non-radiation rate, in favor of the enhancement of RTP efficiency. Furthermore, the introduction of phenylene into the 2-position substitution greatly suppresses the non-radiation, resulting in the simultaneously improved RTP efficiency and elongated lifetime. Finally, using these RTP materials, the dynamically reversible operations of information (write-read-erase) are realized, as well as the encryption and time-dependent decryption demonstration. This work not only provides a better understanding of structure–property relationship on TA-based RTP materials, but also suggests an intramolecular structural modification strategy to improve the performance of pure organic RTP materials.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500024-sup-0001-supinfo.pdfPDF document, 2.4 MB |
Appendix S1: Supporting Information |
| cjoc202500024-sup-0002-supinfo.mp4MPEG-4 video, 41.5 MB |
Appendix S2: Supporting Information |
| cjoc202500024-sup-0003-supinfo.mp4MPEG-4 video, 66.5 MB |
Appendix S3: Supporting Information |
| cjoc202500024-sup-0004-supinfo.mp4MPEG-4 video, 12.8 MB |
Appendix S4: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Higginbotham, H. F.; Okazaki, M.; de Silva, P.; Minakata, S.; Takeda, Y.; Data, P. Heavy-Atom-Free Room-Temperature Phosphorescent Organic Light-Emitting Diodes Enabled by Excited States Engineering. ACS Appl. Mater. Interfaces 2021, 13, 2899–2907.
- 2 Cui, D.; Zhang, L.; Zhang, J.; Li, W.; Chen, J.; Guo, Z.; Sun, C.; Wang, Y.; Wang, W.; Li, S.; Huang, W.; Zheng, C.; Chen, R. Hybrid Local and Charge-Transfer Material with Ultralong Room Temperature Phosphorescence for Efficient Organic Afterglow Light-Emitting Diodes. Angew. Chem. Int. Ed. 2024, 63, e202411588.
- 3 Chen, Z.; Li, M.; Gu, Q.; Peng, X.; Qiu, W.; Xie, W.; Liu, D.; Jiao, Y.; Liu, K.; Zhou, J.; Su, S. J. Highly Efficient Purely Organic Phosphorescence Light-Emitting Diodes Employing a Donor-Acceptor Skeleton with a Phenoxaselenine Donor. Adv. Sci. 2023, 10, e2207003.
- 4 Han, X.; Wang, X.; Wu, Y.; Zhao, J.; Liu, Y.; Shu, H.; Wu, X.; Tong, H.; Wang, L. Modulation of Triplet-Mediated Emission from Selenoxanthen-9-One-Based D–A–D Type Emitters through Tuning the Twist Angle to Realize Electroluminescence Efficiency over 25%. J. Mater. Chem. C 2022, 10, 7437–7442.
- 5 Fan, Y.; Liu, S.; Wu, M.; Xiao, L.; Fan, Y.; Han, M.; Chang, K.; Zhang, Y.; Zhen, X.; Li, Q.; Li, Z. Mobile Phone Flashlight-Excited Red Afterglow Bioimaging. Adv. Mater. 2022, 34, e2201280.
- 6 Cheng, J.; Sun, H.; Zhou, L.; Baryshnikov, G. V.; Liu, M.; Shen, S.; Ågren, H.; Zhu, L. Electrostatic Interaction-Mediated 1:1 Complexes for High-Contrast Mitochondrial-Targeted Phosphorescence Bioimaging. Sci. China Chem. 2024, 67, 3406–3413.
- 7 Gao, H.; Zhang, T.; Lei, Y.; Jiao, D.; Yu, B.; Yuan, W. Z.; Ji, J.; Jin, Q.; Ding, D. An Organophosphorescence Probe with Ultralong Lifetime and Intrinsic Tissue Selectivity for Specific Tumor Imaging and Guided Tumor Surgery. Angew. Chem. Int. Ed. 2024, 63, e202406651.
- 8 Xu, L.; Zhou, K.; Ma, H.; Lv, A.; Pei, D.; Li, G.; Zhang, Y.; An, Z.; Li, A.; He, G. Ultralong Organic Phosphorescent Nanocrystals with Long- Lived Triplet Excited States for Afterglow Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 18385–18394.
- 9 Fu, S.; Chen, Y.; Xie, Y.; Li, Z. Photoactivated Circularly Polarized Room-Temperature Phosphorescence from Phenoselenazine Derivative and Its Application in Information Security. Chin. J. Chem. 2024, 42, 2499–2506.
- 10 Guo, W.-J.; Chen, Y.-Z.; Tung, C.-H.; Wu, L.-Z. Ultralong Room-Temperature Phosphorescence of Silicon-Based Pure Organic Crystal for Oxygen Sensing. CCS Chem. 2022, 4, 1007–1015.
- 11 Liu, H.; Pan, G.; Yang, Z.; Wen, Y.; Zhang, X.; Zhang, S.-T.; Li, W.; Yang, B. Dual-Emission of Fluorescence and Room-Temperature Phosphorescence for Ratiometric and Colorimetric Oxygen Sensing and Detection Based on Dispersion of Pure Organic Thianthrene Dimer in Polymer Host. Adv. Opt. Mater. 2022, 10, 2102814.
- 12 Hamzehpoor, E.; Ruchlin, C.; Tao, Y.; Liu, C. H.; Titi, H. M.; Perepichka, D. F. Efficient Room-Temperature Phosphorescence of Covalent Organic Frameworks through Covalent Halogen Doping. Nat. Chem. 2023, 15, 83–90.
- 13 Zhao, S.; Yang, Z.; Zhang, X.; Liu, H.; Lv, Y.; Wang, S.; Yang, Z.; Zhang, S. T.; Yang, B. A Functional Unit Combination Strategy for Enhancing Red Room-Temperature Phosphorescence. Chem. Sci. 2023, 14, 9733–9743.
- 14 Lewis, G. N.; Kasha, M. Phosphorescence and the Triplet State. J. Am. Chem. Soc. 1944, 66, 2100–2116.
- 15 Baryshnikov, G.; Minaev, B.; Agren, H. Theory and Calculation of the Phosphorescence Phenomenon. Chem. Rev. 2017, 117, 6500–6537.
- 16 Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet Photosensitizers: From Molecular Design to Applications. Chem. Soc. Rev. 2013, 42, 5323–5351.
- 17 Liu, H.; Gao, Y.; Cao, J.; Li, T.; Wen, Y.; Ge, Y.; Zhang, L.; Pan, G.; Zhou, T.; Yang, B. Efficient Room-Temperature Phosphorescence Based on a Pure Organic Sulfur-Containing Heterocycle: Folding-Induced Spin–Orbit Coupling Enhancement. Mater. Chem. Front. 2018, 2, 1853–1858.
- 18 Hirata, S. Recent Advances in Materials with Room-Temperature Phosphorescence: Photophysics for Triplet Exciton Stabilization. Adv. Opt. Mater. 2017, 5, 1700116.
- 19 Ju, C. W.; Wang, X. C.; Li, B.; Ma, Q.; Shi, Y.; Zhang, J.; Xu, Y.; Peng, Q.; Zhao, D. Evolution of Organic Phosphor through Precision Regulation of Nonradiative Decay. Proc. Natl. Acad. Sci. U. S. A. 2023, 120, e2310883120.
- 20 Yang, Y.; Yang, X.; Fang, X.; Wang, K. Z.; Yan, D. Reversible Mechanochromic Delayed Fluorescence in 2D Metal-Organic Micro/Nanosheets: Switching Singlet-Triplet States through Transformation between Exciplex and Excimer. Adv. Sci. 2018, 5, 1801187.
- 21 Li, Y.; Jiang, L.; Liu, W.; Xu, S.; Li, T. Y.; Fries, F.; Zeika, O.; Zou, Y.; Ramanan, C.; Lenk, S.; Scholz, R.; Andrienko, D.; Feng, X.; Leo, K.; Reineke, S. Reduced Intrinsic Non-Radiative Losses Allow Room-Temperature Triplet Emission from Purely Organic Emitters. Adv. Mater. 2021, 33, e2101844.
- 22 Pattanayak, P.; Nandi, A.; Patra, R.; Purkayastha, P. Triplet Exciton Harvesting in Water by a Structurally Simple Organic Phosphor with Sustained Afterglow and Lifetime Tunability. Adv. Opt. Mater. 2024, 12, 2302155.
- 23 Gu, L.; Wu, H.; Ma, H.; Ye, W.; Jia, W.; Wang, H.; Chen, H.; Zhang, N.; Wang, D.; Qian, C.; An, Z.; Huang, W.; Zhao, Y. Color-Tunable Ultralong Organic Room Temperature Phosphorescence from a Multicomponent Copolymer. Nat. Commun. 2020, 11, 944.
- 24 Xiao, L.; Fu, H. Enhanced Room-Temperature Phosphorescence through Intermolecular Halogen/Hydrogen Bonding. Chem. - Eur. J 2019, 25, 714–723.
- 25 Liu, Q.; Liu, X.; Yu, X.; Zhang, X.; Zhu, M.; Cheng, Y. Circularly Polarized Room Temperature Phosphorescence through Twisting-Induced Helical Structures from Polyvinyl Alcohol-Based Fibers Containing Hydrogen-Bonded Dyes. Angew. Chem. Int. Ed. 2024, 63, e202403391.
- 26 Ma, L. W.; Ma, X. Recent Advances in Room-Temperature Phosphorescent Materials by Manipulating Intermolecular Interactions. Sci. China Chem. 2023, 66, 304–314.
- 27 Zhang, Y.; Chen, J.; Sun, Q.; Zhang, H.; Xue, S.; Yang, W. In-Situ Grafting N-Arylcarbazoles Enables More Ultra-Long Room Temperature Phosphorescence Polymers. Chem. Eng. J. 2023, 452, 139385.
- 28 Wang, S.; Ma, L.; Wang, Q.; Shao, P.; Ma, D.; Yuan, S.; Lei, P.; Li, P.; Feng, X.; Wang, B. Covalent Organic Frameworks: A Platform for the Experimental Establishment of the Influence of Intermolecular Distance on Phosphorescence. J. Mater. Chem. C 2018, 6, 5369–5374.
- 29 Jiang, J.; Du, X.; Zhang, K. Achieving Ultralong Room-Temperature Phosphorescence in Covalent Organic Framework System. J. Phys. Chem. Lett. 2024, 15, 1658–1667.
- 30 Lei, X.; Wang, J.; Zhao, H.; Yang, T.; Bai, G.; Feng, X.; Zhou, Q.; Yi, L.; Yuan, W. Z. Unexpected Tunable Photoluminescence and Emission Mechanism during the Gradual Increase of Cyclic Glucose Units. Chem. Eng. J. 2024, 496, 154155.
- 31 Lin, X.; Xu, Q.; Ma, X. Emission-Tunable Room-Temperature Phosphorescent Polymers Based on Dynamic Reversible Supramolecule- Mediated Photocrosslinking. Adv. Opt. Mater. 2022, 10, 2101646.
- 32 Xu, D.-A.; Zhou, Q.-Y.; Dai, X.; Ma, X.-K.; Zhang, Y.-M.; Xu, X.; Liu, Y. Cucurbit[8]Uril-Mediated Phosphorescent Supramolecular Foldamer for Antibiotics Sensing in Water and Cells. Chin. Chem. Lett. 2022, 33, 851–854.
- 33 Demangeat, C.; Dou, Y.; Hu, B.; Bretonniere, Y.; Andraud, C.; D'Aleo, A.; Wu, J. W.; Kim, E.; Le Bahers, T.; Attias, A. J. Sigma-Conjugation and H-Bond-Directed Supramolecular Self-Assembly: Key Features for Efficient Long-Lived Room Temperature Phosphorescent Organic Molecular Crystals. Angew. Chem. Int. Ed. 2021, 60, 2446–2454.
- 34 Zhang, W.; Luo, Y.; Liu, C.; Yang, M. X.; Gou, J. X.; Huang, Y.; Ni, X. L.; Tao, Z.; Xiao, X. Supramolecular Room Temperature Phosphorescent Materials Based on Cucurbit[8]Uril for Dual Detection of Dodine. ACS Appl. Mater. Interfaces 2022, 14, 51429–51437.
- 35 Zhou, W.-L.; Lin, W.; Chen, Y.; Liu, Y. Supramolecular Assembly Confined Purely Organic Room Temperature Phosphorescence and Its Biological Imaging. Chem. Sci. 2022, 13, 7976–7989.
- 36 Xie, Z.; Zhang, X.; Wang, H.; Huang, C.; Sun, H.; Dong, M.; Ji, L.; An, Z.; Yu, T.; Huang, W. Wide-Range Lifetime-Tunable and Responsive Ultralong Organic Phosphorescent Multi-Host/Guest System. Nat. Commun. 2021, 12, 3522.
- 37 Xia, Y.; Zhu, C.; Cao, F.; Shen, Y.; Ouyang, M.; Zhang, Y. Host-Guest Doping in Flexible Organic Crystals for Room-Temperature Phosphorescence. Angew. Chem. Int. Ed. 2023, 62, e202217547.
- 38 Guo, S.; Dai, W.; Chen, X.; Lei, Y.; Shi, J.; Tong, B.; Cai, Z.; Dong, Y. Recent Progress in Pure Organic Room Temperature Phosphorescence of Small Molecular Host–Guest Systems. ACS Mater. Lett. 2021, 3, 379–397.
- 39 Law, A. W. K.; Cheung, T. S.; Zhang, J.; Leung, N. L. C.; Kwok, R. T. K.; Zhao, Z.; Sung, H. H. Y.; Williams, I. D.; Qiu, Z.; Alam, P.; Lam, J. W. Y.; Tang, B. Z. Sergeant-and-Soldier Effect in an Organic Room-Temperature Phosphorescent Host-Guest System. Adv. Mater. 2024, 36, e2410739.
- 40 Deng, H.; Li, G.; Xie, H.; Yang, Z.; Mao, Z.; Zhao, J.; Yang, Z.; Zhang, Y.; Chi, Z. Dynamic Ultra-Long Room Temperature Phosphorescence Enabled by Amorphous Molecular "Triplet Exciton Pump" for Encryption with Temporospatial Resolution. Angew. Chem. Int. Ed. 2024, 63, e202317631.
- 41 Wang, X.; Wang, Z.; Feng, H.; Lin, C.; Shi, H.; An, Z.; Su, Z. M.; Liang, F. S. Activating Room-Temperature Phosphorescence of 1,8-Naphthalimide by Doping into Aromatic Dicarboxylic Acids. Chem. Commun. 2022, 58, 3641–3644.
- 42 Si, Y.; Zhao, Y.; Dai, W.; Cui, S.; Sun, P.; Shi, J.; Tong, B.; Cai, Z.; Dong, Y. Organic Host-Guest Materials with Bright Red Room-Temperature Phosphorescence for Persistent Bioimaging. Chin. J. Chem. 2023, 41, 1575–1582.
- 43 Russo, R.; Chotard, J.-N.; Gachot, G.; Frayret, C.; Toussaint, G.; Stevens, P.; Becuwe, M. High-Output-Voltage and -Energy-Density All-Organic Dual-Ion Battery Using Molecular Thianthrene. ACS Energy Lett. 2023, 8, 4597–4607.
- 44 Fiedler, B.; Reckien, W.; Bredow, T.; Beck, J.; Sokolowski, M. Structure and Charge Transfer in Binary Ordered Monolayers of Two Sulfur-Containing Donor Molecules and Tnap on the Au(111) Surface. J. Phys. Chem. C 2014, 118, 3035–3048.
- 45 Riebe, S.; Adam, S.; Roy, B.; Maisuls, I.; Daniliuc, C. G.; Dubbert, J.; Strassert, C. A.; Schapiro, I.; Voskuhl, J. Bridged Aromatic Oxo- and Thioethers with Intense Emission in Solution and the Solid State. Chem. Asian J. 2021, 16, 2307–2313.
- 46 Li, N.; Wang, Y.; Li, Z. Photo-Induced Room Temperature Phosphorescence and Thermally Activated Photochromism Based on Thianthrene Derivatives. J. Mater. Chem. C 2024, 12, 12045–12053.
- 47 Wang, H.; Hao, F.; Ba, Z.; Xiao, Y.; Yu, T. Realizing Mechano-Tunable Dual Emission in a Twisted Thianthrene Derivative. Dyes Pigm. 2024, 223, 111932.
- 48 Pan, G.; Yang, Z.; Liu, H.; Wen, Y.; Zhang, X.; Shen, Y.; Zhou, C.; Zhang, S. T.; Yang, B. Folding-Induced Spin-Orbit Coupling Enhancement for Efficient Pure Organic Room-Temperature Phosphorescence. J. Phys. Chem. Lett. 2022, 13, 1563–1570.
- 49 Yang, Z.; Fu, Z.; Liu, H.; Wu, M.; Li, N.; Wang, K.; Zhang, S. T.; Zou, B.; Yang, B. Pressure-Induced Room-Temperature Phosphorescence Enhancement Based on Purely Organic Molecules with a Folded Geometry. Chem. Sci. 2023, 14, 2640–2645.
- 50 Yang, Z.; Liu, H.; Zhang, X.; Lv, Y.; Fu, Z.; Zhao, S.; Liu, M.; Zhang, S. T.; Yang, B. Photo-Responsive Dynamic Organic Room-Temperature Phosphorescence Materials Based on a Functional Unit Combination Strategy. Adv. Mater. 2024, 36, e2306784.
- 51 Yang, Z.; Zhao, S.; Zhang, X.; Liu, M.; Liu, H.; Yang, B. Efficient Room-Temperature Phosphorescence from Discrete Molecules Based on Thianthrene Derivatives for Oxygen Sensing and Detection. Front. Chem. 2021, 9, 810304.
- 52 Liu, M.; Yang, Z.; Feng, Z.; Zhao, N.; Bian, R.; Wu, J.; Yang, Q.; Zhao, S.; Liu, H.; Yang, B. Combining Functional Units to Design Organic Materials with Dynamic Room-Temperature Phosphorescence under Continuous Ultraviolet Irradiation. Molecules 2024, 29, 2621.
- 53 Leitonas, K.; Tomkeviciene, A.; Baratte, G.; Dabuliene, A.; Punniyakoti, S. M.; Volyniuk, D.; Grazulevicius, J. V. Oxygen Sensing Properties of Thianthrene and Phenothiazine Derivatives Exhibiting Room Temperature Phosphorescence: Effect of Substitution of Phenothiazine Moieties. Sens. Actuators, B 2021, 345, 130369.
- 54 Volyniuk, L.; Gudeika, D.; Panchenko, A. A.; Minaev, B. F.; Mahmoudi, M.; Simokaitiene, J.; Bucinskas, A.; Volyniuk, D.; Grazulevicius, J. V. Single-Molecular White Emission of Organic Thianthrene-Based Luminophores Exhibiting Efficient Fluorescence and Room Temperature Phosphorescence Induced by Halogen Atoms. ACS Sustainable Chem. Eng. 2023, 11, 16914–16925.
- 55 Li, M.; Xie, W.; Cai, X.; Peng, X.; Liu, K.; Gu, Q.; Zhou, J.; Qiu, W.; Chen, Z.; Gan, Y.; Su, S.-J. Molecular Engineering of Sulfur-Bridged Polycyclic Emitters Towards Tunable TADF and RTP Electroluminescence. Angew. Chem. Int. Ed. 2022, 61, e202209343.
- 56 Liu, W.; Wang, F.; Li, L. The Beijing Density Functional (Bdf) Program Package: Methodologies and Applications. J. Theor. Comput. Chem. 2003, 02, 257–272.
- 57 Liu, W.; Hong, G.; Dai, D.; Li, L.; Dolg, M. The Beijing Four-Component Density Functional Program Package (BDF) and Its Application to Euo, Eus, Ybo and Ybs. Theor. Chem. Acc. 1997, 96, 75–83.
- 58 Zhang, Y.; Suo, B.; Wang, Z.; Zhang, N.; Li, Z.; Lei, Y.; Zou, W.; Gao, J.; Peng, D.; Pu, Z.; Xiao, Y.; Sun, Q.; Wang, F.; Ma, Y.; Wang, X.; Guo, Y.; Liu, W. Bdf: A Relativistic Electronic Structure Program Package. J. Chem. Phys. 2020, 152, 064113.
- 59 Li, Z.; Suo, B.; Zhang, Y.; Xiao, Y.; Liu, W. Combining Spin-Adapted Open-Shell TD-DFT with Spin–Orbit Coupling. Mol. Phys. 2013, 111, 3741–3755.
- 60 Li, Z.; Xiao, Y.; Liu, W. On the Spin Separation of Algebraic Two-Component Relativistic Hamiltonians. J. Chem. Phys. 2012, 137, 154114.
- 61 Li, Z.; Xiao, Y.; Liu, W. On the Spin Separation of Algebraic Two-Component Relativistic Hamiltonians: Molecular Properties. J. Chem. Phys. 2014, 141, 054111.
- 62
Ma, H.; Lv, A.; Fu, L.; Wang, S.; An, Z.; Shi, H.; Huang, W. Room-Temperature Phosphorescence in Metal-Free Organic Materials. Ann. Phys. 2019, 531, 1800482.
10.1002/andp.201800482 Google Scholar