Enantioselective Synthesis of Planar/Multiple Chiral [n]Cyclophanes through Asymmetric Allylation
Ziyang Wang
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorXin-Xin Zhang
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYidan Sun
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Hanliang Zheng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xin Li
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300192 China
E-mail: [email protected]; [email protected]Search for more papers by this authorZiyang Wang
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorXin-Xin Zhang
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYidan Sun
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Hanliang Zheng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xin Li
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300192 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
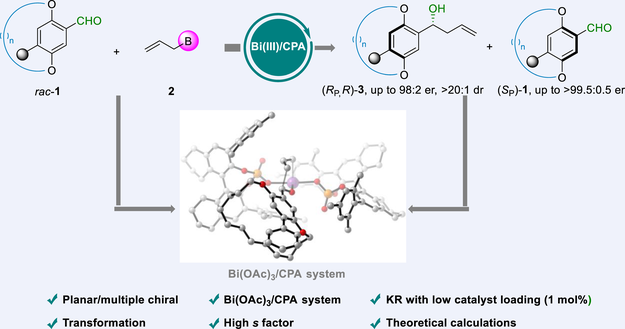
Planar-chiral cyclophanes with carbon-centered chirality are important targets in natural products and pharmaceuticals. However, synthesizing such planar chiral cyclophanes with two stereogenic elements via a one-step asymmetric reaction remains a formidable challenge. Herein, we present an efficient kinetic resolution method for synthesizing planar-chiral [n]cyclophanes with carbon-centered chirality. This is achieved through the enantioselective allylation of racemic aldehyde [n]cyclophanes catalyzed by Bi(OAc)3 and chiral phosphoric acid. The reaction delivers planar-chiral [n]cyclophanes and multiple chiral [n]cyclophanes with high yields and excellent enantioselectivities, showcasing remarkable kinetic resolution efficiency (s factor up to 292). The broad substrate scope, scalability, and potential for derivatization highlight the value of this methodology. DFT calculations have also been performed to provide insights into the origin of the experimentally observed diastereo- and enantioselectivity for this reaction.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500010-sup-0001-supinfo.pdfPDF document, 25.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 López, R.; Palomo, C. Planar Chirality: A Mine for Catalysis and Structure Discovery. Angew. Chem. Int. Ed. 2022, 61, e202113504.
- 2 Lüttringhaus, A.; Gralheer, H. Über Eine Neue Art Atropisomerer Verbindungen. Justus Liebigs Ann. Chem. 1942, 550, 67–98.
- 3(a) Fu, X.; Hossain, M. B.; van der Helm, D.; Schmitz, F. J. Longithorone A: Unprecedented Dimeric Prenylated Quinone from the Tunicate Aplydium Longithorax. J. Am. Chem. Soc. 1994, 116, 12125–12126; (b) Bartholomew, G. P.; Bazan, G. C. Bichromophoric Paracyclophanes: Models for Interchromophore Delocalization. Acc. Chem. Res. 2001, 34, 30–39; (c) Bartholomew, G. P.; Ledoux, I.; Mukamel, S.; Bazan, G. C.; Zyss, J. Three-Dimensional Nonlinear Optical Chromophores Based on Through-Space Delocalization. J. Am. Chem. Soc. 2002, 124, 13480–13485; (d) Hopf, H. [2.2]Paracyclophanes in Polymer Chemistry and Materials Science. Angew. Chem. Int. Ed. 2008, 47, 9808–9812; (e) Gulder, T.; Baran, P. S. Strained Cyclophane Natural Products: Macrocyclization at Its Limits. Nat. Prod. Rep. 2012, 29, 899–934; (f) Takiguchi, H.; Ohmori, K.; Suzuki, K. Synthesis and Determination of the Absolute Configuration of Cavicularin by a Symmetrization/Asymmetrization Approach. Angew. Chem. Int. Ed. 2013, 52, 10472–10476; (g) Glunz, P. W.; Mueller, L.; Cheney, D. L.; Ladziata, V.; Zou, Y.; Wurtz, N. R.; Wei, A.; Wong, P. C.; Wexler, R. R.; Priestley, E. S. Atropisomer Control in Macrocyclic Factor VIIa Inhibitors. J. Med. Chem. 2016, 59, 4007–4018; (h) Glunz, P. W. Recent Encounters with Atropisomerism in Drug Discovery. Med. Chem. Lett. 2018, 28, 53–60; (i) Syed, Y. Y. Lorlatinib: First Global Approval. Drugs 2019, 79, 93–98; (j) Smolyar, I. V.; Yudin, A. K.; Nenajdenko, V. G. Heteroaryl Rings in Peptide Macrocycles. Chem. Rev. 2019, 119, 10032–10240; (k) Wang, Y.; Joullié, M. M. Approaches to Cyclophane-Types of Cyclopeptide Alkaloids. Chem. Rec. 2021, 21, 906–923; (l) Trofimova, A.; Diamandas, M.; Brien, C.; Khasanzoda, N.; Lough, A. J.; Yudin, A. K. Terpenoid Cyclophanes with Planar Chirality. J. Am. Chem. Soc. 2024, 146, 23365–23375.
- 4For selected reviews, see: (a) Cheng, H.-G.; Jia, S.; Zhou, Q. Benzo- Fused-Ring Toolbox Based on Palladium/Norbornene Cooperative Catalysis: Methodology Development and Applications in Natural Product Synthesis. Acc. Chem. Res. 2023, 56, 573–591; (b) Zhang, H.-H.; Li, T.-Z.; Liu, S.-J.; Shi, F. Catalytic Asymmetric Synthesis of Atropisomers Bearing Multiple Chiral Elements: An Emerging Field. Angew. Chem. Int. Ed. 2024, 63, e202311053. For selected examlpes, see: (c) Jia, S.; Li, S.; Liu, Y.; Qin, W.; Yan, H. Enantioselective Control of Both Helical and Axial Stereogenic Elements Though an Organocatalytic Approach. Angew. Chem. Int. Ed. 2019, 58, 18496–18501; (d) Ma, C.; Sheng, F.-T.; Wang, H.-Q.; Deng, S.; Zhang, Y.-C.; Jiao, Y.-C.; Tan, W.; Shi, F. Atroposelective Access to Oxindole-Based Axially Chiral Styrenes via the Strategy of Catalytic Kinetic Resolution. J. Am. Chem. Soc. 2020, 142, 15686–15696; (e) Huang, S.; Wen, H.; Tian, Y.; Wang, P.; Qin, W.; Yan, H. Organocatalytic Enantioselective Construction of Chiral Azepine Skeleton Bearing Multiple-Stereogenic Elements. Angew. Chem. Int. Ed. 2021, 60, 21486–21493; (f) Chen, Y.; He, J.; Zhuang, C.; Liu, Z.; Xiao, K.; Su, Z.; Ren, X.; Wang, T. Synergistic Catalysis Between a Dipeptide Phosphonium Salt and a Metal-Based Lewis Acid for Asymmetric Synthesis of N-Bridged [3.2.1] Ring Systems. Angew. Chem. Int. Ed. 2022, 61, e202207334; (g) Wu, J.-H.; Fang, S.; Zheng, X.; He, J.; Ma, Y.; Su, Z.; Wang, T. Organocatalytic Dynamic Kinetic Resolution Enabled Asymmetric Synthesis of Phosphorus-Containing Chiral Helicenes. Angew. Chem. Int. Ed. 2023, 62, e202309515; (h) Ye, J.; Li, L.; You, Y.; Jiao, C.; Cui, Z.; Zhang, Y.; Jia, S.; Cong, H.; Liu, S.; Cheng, H.-G.; Zhou, Q. Enantioselective Assembly of Ferrocenes with Axial and Planar Chiralities via Palladium/Chiral Norbornene Cooperative Catalysis. JACS Au 2023, 3, 384–390; (i) Shen, G.; He, F.; Xie, W.; Gu, H.; Yang, X. Diastereodivergent Asymmetric [4 + 2] Cycloaddition of In Situ Generated ortho-Quinone Methides and Allenyl Ketones Enabled by Chiral Phosphoric Acid Catalysis. ACS Catal. 2023, 13, 12472−12480; (j) Wang, J.-Y.; Gao, C.-H.; Ma, C.; Wu, X.-Y.; Ni, S.-F.; Tan, W.; Shi, F. Design and Catalytic Asymmetric Synthesis of Furan-Indole Compounds Bearing Both Axial and Central Chirality. Angew. Chem. Int. Ed. 2024, 63, e202316454; (k) Ye, Z.; Xie, W.; Wang, D.; Liu, H.; Yang, X. Atroposelective Synthesis of Diarylamines via Organocatalyzed Electrophilic Amination. ACS Catal. 2024, 14, 4958–4967.
- 5For selected reviews, see: (a) Kotha, S.; Shirbhate, M. E.; Waghule, G. T. Selected Synthetic Strategies to Cyclophanes. Beilstein J. Org. Chem. 2015, 11, 1274–1331;
(b) Li, J.; Huang, D.; Fang, Q.; Zhao, C. Progress on Synthesis of Optically Pure Mechanical Planar Chiral Rotaxanes and Planar Chiral Macrocycle Molecules. Chin. Sci. Bull. 2022, 67, 2383–2392;
10.1360/TB-2021-1324 Google Scholar(c) Yang, G.; Wang, J. Recent Advances on Catalytic Atroposelective Synthesis of Planar-Chiral Macrocycles. Angew. Chem. Int. Ed. 2024, 63, e202412805; (d) Dong, Z.; Li, J.; Zhao, C. Catalytic Enantioselective Macrocyclization for the Synthesis of Planar- Chiral Cyclophanes: Recent Updates. Eur. J. Org. Chem. 2024, e202400841; (e) Zhao, Y.-H.; Zhu, D.; Chen, Z.-M. Enantioselective Construction of Planar-Chiral Molecules by Catalytic Asymmetric Late-Stage Functionalizations. ChemCatChem 2024, 16, e202401312; (f) Gaucherand, A.; Yen-Pon, E.; Domain, A.; Bourhis, A.; Rodriguez, J.; Bonne, D. Enantioselective Synthesis of Molecules with Multiple Stereogenic Elements. Chem. Soc. Rev. 2024, 53, 11165–11206
- 6(a) Tanaka, K.; Sagae, H.; Toyoda, K.; Noguchi, K.; Hirano, M. Enantioselective Synthesis of Planar-Chiral Metacyclophanes through Rhodium-Catalyzed Alkyne Cyclotrimerization. J. Am. Chem. Soc. 2007, 129, 1522–1523; (b) Tanaka, K.; Sagae, H.; Toyoda, K.; Hirano, M. Enantioselective Synthesis of Planar-Chiral Metacyclophanes through Cationic Rh(I)/Modified-BINAP-Catalyzed Inter- and Intramolecular Alkyne Cyclotrimerizations. Tetrahedron 2008, 64, 831–846; (c) Shibata, T.; Uchiyama, T.; Endo, K. Enantioselective Synthesis of Chiral Tripodal Cage Compounds by [2 + 2 + 2] Cycloaddition of Branched Triynes. Org. Lett. 2009, 11, 3906–3908; (d) Araki, T.; Hojo, D.; Noguchi, K.; Tanaka, K. Enantioselective Synthesis of Planar Chiral Paracyclophanes with Short Ansa Chains and Structure of Strained Dioxa[7]Paracyclophane. Synlett 2011, 4, 539–542; (e) Araki, T.; Noguchi, K.; Tanaka, K. Enantioselective Synthesis of Planar-Chiral Carba-Paracyclophanes: Rhodium-Catalyzed [2+2+2] Cycloaddition of Cyclic Diynes with Terminal Monoynes. Angew. Chem. Int. Ed. 2013, 52, 5617–5621; (f) Nogami, J.; Tanaka, Y.; Sugiyama, H.; Uekusa, H.; Muranaka, A.; Uchiyama, M.; Tanaka, K. Enantioselective Synthesis of Planar Chiral Zigzag-Type Cyclophenylene Belts by Rhodium-Catalyzed Alkyne Cyclotrimerization. J. Am. Chem. Soc. 2020, 142, 9834–9842; (g) Nogami, J.; Nagashima, Y.; Miyamoto, K.; Muranaka, A.; Uchiyama, M.; Tanaka, K. Asymmetric Synthesis, Structures, and Chiroptical Properties of Helical Cycloparaphenylenes. Chem. Sci. 2021, 12, 7858–7865.
- 7(a) Kanda, K.; Koike, T.; Endo, K.; Shibata, T. The First Asymmetric Sonogashira Coupling for the Enantioselective Generation of Planar Chirality in Paracyclophanes. Chem. Commun. 2009, 14, 1870–1872;
10.1039/b818904h Google Scholar(b) Kanda, K.; Endo, K.; Shibata, T. Enantioselective Synthesis of Planar-Chiral 1,n-Dioxa[n]Paracyclophanes via Catalytic Asymmetric Ortho-Lithiation. Org. Lett. 2010, 12, 1980–1983; (c) Kanda, K.; Hamanaka, R.; Endo, K.; Shibata, T. Asymmetric Ortho-Lithiation of 1,n-Dioxa[n]Paracyclophane Derivatives for the Generation of Planar Chirality. Tetrahedron 2012, 68, 1407–1416; (d) Kanda, K.; Oshima, S.; Shizuno, T.; Hamanaka, R.; Fukai, M.; Shibata, T. Enantioselective Synthesis of Planar-Chiral Phosphines with 1,n-Dioxa[n]Paracyclophane Scaffold and Their Application as Chiral Ligands. Heterocycles 2014, 88, 1355–1370; (e) Hazra, M.; Kanyiva, K. S.; Shibata, T. Enantioselective Synthesis of Planar-Chiral 1,11-Dioxa[11]Paracyclophane- Derived Phosphoramidites and Their Use as Chiral Ligands. Tetrahedron: Asymmetry 2016, 27, 1081–1087; (f) Shibata, T.; Fukai, M.; Sekine, R.; Hazra, M.; Kanyiva, K. S. Enantioselective Synthesis of Planar-Chiral 1,n-Dioxa[n]Paracyclophane-Based Phosphites and Their Application as Chiral Ligands. Synthesis 2016, 48, 2664–2670; (g) Delcourt, M.-L.; Felder, S.; Benedetti, E.; Micouin, L. Highly Enantioselective Desymmetrization of Centrosymmetric Pseudo-Para-Diformyl[2.2]Paracyclophane via Asymmetric Transfer Hydrogenation. ACS Catal. 2018, 8, 6612–6616; (h) Wang, D.; Shao, Y.-B.; Chen, Y.; Xue, X.-S.; Yang, X. Enantioselective Synthesis of Planar-Chiral Macrocycles through Asymmetric Electrophilic Aromatic Amination. Angew. Chem. Int. Ed. 2022, 134, e202201064;10.1002/ange.202201064 Google Scholar(i) Dong, Z.; Li, J.; Yao, T.; Zhao, C. Palladium-Catalyzed Enantioselective C−H Olefination to Access Planar-Chiral Cyclophanes by Dynamic Kinetic Resolution. Angew. Chem. Int. Ed. 2023, 62, e202315603; (j) Zhu, D.; Mu, T.; Li, Z.-L.; Luo, H.-Y.; Cao, R.-F.; Xue, X.-S.; Chen, Z.-M. Enantioselective Synthesis of Planar-Chiral Sulfur-Containing Cyclophanes by Chiral Sulfide Catalyzed Electrophilic Sulfenylation of Arenes. Angew. Chem. Int. Ed. 2024, 63, e202318625; (k) Li, J.; Dong, Z.; Chen, Y.; Yang, Z.; Yan, X.; Wang, M.; Li, C.; Zhao, C. N-Heterocyclic Carbene-Catalyzed Enantioselective Synthesis of Planar-Chiral Cyclophanes via Dynamic Kinetic Resolution. Nat. Commun. 2024, 15, 2338; (l) Dočekal, V.; Koucký, F.; Císařová, I.; Veselý, J. Organocatalytic Desymmetrization Provides Access to Planar Chiral [2.2]Paracyclophanes. Nat. Commun. 2024, 15, 3090; (m) Li, J.; Dong, Z.; Zhai, H.; Wu, J.; Zhao, C. An Approach for Highly Enantioselective Synthesis of meta-Disubstituted [n]Paracyclophanes. J. Org. Chem. 2024, 89, 15374–15379.
- 8(a) Islas-Gonzalez, G.; Bois-Choussy, M.; Zhu, J. From Central to Planar Chirality, the First Example of Atropenantioselective Cycloetherification. Org. Biomol. Chem. 2003, 1, 30–32; (b) Tanaka, K.; Hori, T.; Osaka, T.; Noguchi, K.; Hirano, M. Rhodium-Catalyzed Reactions of Dithiols and 1,4-Bis(bromomethyl)benzenes Leading to Enantioenriched Dithiaparacyclophanes. Org. Lett. 2007, 9, 4881–4884; (c) Mori, K.; Ohmori, K.; Suzuki, K. Hydrogen-Bond Control in Axially Chiral Styrenes: Selective Synthesis of Enantiomerically Pure C2-Symmetric Paracyclophanes. Angew. Chem. Int. Ed. 2009, 48, 5638–5641; (d) Ranyuk, E. R.; Averin, A. D.; Beletskaya, I. P. One-Step Synthesis of Chiral Azamacrocycles via Palladium-Catalyzed Enantioselective Amination of 1,5-Dichloroanthraquinone and 1,5-Dichloroanthracene. Adv. Synth. Catal. 2010, 352, 2299–2305; (e) Hori, T.; Shibata, Y.; Tanaka, K. Asymmetric Synthesis of Planar-Chiral Paracyclophanes by Double C–S Bond Formation: Comparison of Catalytic Activity and Enantioselectivity of Pd and Rh Catalysts. Tetrahedron: Asymmetry 2010, 21, 1303–1306; (f) Salih, M. Q.; Beaudry, C. M. Enantioselective Ullmann Ether Couplings: Syntheses of (−)-Myricatomentogenin, (−)-Jugcathanin, (+)-Galeon, and (+)-Pterocarine. Org. Lett. 2013, 15, 4540–4543; (g) Zhao, P.; Beaudry, C. M. Enantioselective and Regioselective Pyrone Diels–Alder Reactions of Vinyl Sulfones: Total Synthesis of (+)-Cavicularin. Angew. Chem. Int. Ed. 2014, 53, 10500–10503; (h) Ding, Q.; Wang, Q.; He, H.; Cai, Q. Asymmetric Synthesis of (−)-Pterocarine and (−)-Galeon via Chiral Phase Transfer-Catalyzed Atropselective Formation of Diarylether Cyclophane Skeleton. Org. Lett. 2017, 19, 1804–1807; (i) Gagnon, C.; Godin, É.; Minozzi, C.; Sosoe, J.; Pochet, C.; Collins, S. K. Biocatalytic Synthesis of Planar Chiral Macrocycles. Science 2020, 367, 917–921; (j) Yu, S.; Shen, G.; He, F.; Yang, X. Asymmetric Synthesis of Planar-Chiral Macrocycles via Organocatalyzed Enantioselective Macrocyclization. Chem. Commun. 2022, 58, 7293–7296; (k) Wei, S.; Chen, L.-Y.; Li, J. Enantioselective Synthesis of Planar Chiral Macrocyclic Metacyclophanes by Pd-Catalyzed C–O Cross-Coupling. ACS Catal. 2023, 13, 7450–7456; (l) Yang, G.; He, Y.; Wang, T.; Li, Z.; Wang, J. Atroposelective Synthesis of Planar-Chiral Indoles via Carbene Catalyzed Macrocyclization. Angew. Chem. Int. Ed. 2024, 63, e202316739; (m) Wang, J.; Wang, M.; Wen, Y.; Teng, P.; Li, C.; Zhao, C. N-Heterocyclic Carbene-Catalyzed Highly Enantioselective Macrolactonization to Access Planar-Chiral Macrocycles. Org. Lett. 2024, 26, 1040–1045; (n) Lv, X.; Su, F.; Long, H.; Lu, F.; Zeng, Y.; Liao, M.; Che, F.; Wu, X.; Chi, Y. R. Carbene Organic Catalytic Planar Enantioselective Macrolactonization. Nat. Commun. 2024, 15, 958; (o) Zhai, H.; Lv, K.; Li, J.; Wang, J.; Liu, T.; Zhao, C. Rhodium(III)-Catalyzed Atroposelective Indolization to Access Planar-Chiral Macrocycles. J. Am. Chem. Soc. 2024, 146, 29214–29223; (p) Yang, G.; Liu, S.; Ji, S.; Wu, X.; Wang, J. Pd/NHC Sequentially Catalyzed Atroposelective Synthesis of Planar-Chiral Macrocycles. Chem. Sci. 2024, 15, 19599–19603.
- 9(a) Akagawa, K.; Higuchi, J.; Yoshikawa, I.; Kudo, K. Kinetic Resolution of Ansa Cyclophanes by Peptide-Catalyzed Aldol/Retro-Aldol Reactions. Eur. J. Org. Chem. 2018, 5278–5281; (b) Li, J.; Zhao, C. Highly Enantioselective Synthesis of Planar-Chiral Cyclophanes Through a Brønsted Acid-Catalyzed Asymmetric Transfer Hydrogenation. ACS Catal. 2023, 13, 14155–14162; (c) Tian, J.; Tmaribuchi, K.; Yoshikawa, I.; Kudo, K. Kinetic Resolution of a Planar–Chiral [2.2]Paracyclophane via Michael Addition to Nitroolefins Catalyzed by N-Terminal Guanidinylated Helical Peptide. Eur. J. Org. Chem. 2024, 27, e202400117; (d) Dorizon, P.; Martin, C.; Daran, J.-C.; Fiaud, J.-C.; Kagan, H. B. A Practical Kinetic Resolution of 4-Acetyl[2.2]paracyclophane. Tetrahedron: Asymmetry 2001, 12, 2625–2630; (e) Alba, A.-N.; Gómez-Sal, P.; Rios, R.; Moyano, A. Organocatalytic Kinetic Resolution of a Planar-Chiral Ferrocenecarbaldehyde. Tetrahedron: Asymmetry 2009, 20, 1314–1318; (f) Akiyama, M.; Akagawa, K.; Seino, H.; Kudo, K. Peptide-Catalyzed Kinetic Resolution of Planar-Chiral Metallocenes. Chem. Commun. 2014, 50, 7893–7896; (g) Akagawa, K.; Nishi, N.; Yoshikawa, I.; Kudo, K. Kinetic Resolution of a Planar-Chiral [2.2]Paracyclophane Derivative by Helical-Peptide-Catalyzed Michael Addition of Nitromethane. Eur. J. Org. Chem. 2015, 2015, 5055–5059; (h) Zhao, Y.; Wang, H.; Wu, B.; Zhou, Y.-G. Synthesis of Paracyclophanes with Planar and Central Chirality: Kinetic Resolution of [2.2] Paracyclophane Aldimines via Palladium-Catalyzed Addition of Arylboronic Acids. Org. Chem. Front. 2019, 6, 3956–3960; (i) Hayashi, Y. Time Economy in Total Synthesis. J. Org. Chem. 2021, 86, 1−23.
- 10For selected reviews of asymmetric allylation, see: (a) Hall, D. G. Lewis and Brønsted Acid Catalyzed Allylboration of Carbonyl Compounds: From Discovery to Mechanism and Applications. Synlett 2007, 11, 1644–1655; (b) Yus, M.; Gonzalez-Gomez, J. C.; Foubelo, F. Catalytic Enantioselective Allylation of Carbonyl Compounds and Imines. Chem. Rev. 2011, 111, 7774–7854; (c) Huo, H.-X.; Duvall, J. R.; Huang, M.-Y.; Hong, R. Catalytic Asymmetric Allylation of Carbonyl Compounds and Imines with Allylic Boronates. Org. Chem. Front. 2014, 1, 303–320; (d) Diner, C.; Szabó, K. J. Recent Advances in the Preparation and Application of Allylboron Species in Organic Synthesis. J. Am. Chem. Soc. 2017, 139, 2–14.
- 11For selected asymmetric allylation of aldehydes, see: (a) Ishiyama, T.; Ahiko, T.; Miyaura, N. Acceleration Effect of Lewis Acid in Allylboration of Aldehydes: Catalytic, Regiospecific, Diastereospecific, and Enantioselective Synthesis of Homoallyl Alcohols. J. Am. Chem. Soc. 2002, 124, 12414–12415; (b) Yu, S. H.; Ferguson, M. J.; McDonald, R.; Hall, D. G. Brønsted Acid-Catalyzed Allylboration: Short and Stereodivergent Synthesis of All Four Eupomatilone Diastereomers with Crystallographic Assignments. J. Am. Chem. Soc. 2005, 127, 12808–12809; (c) Rauniyar, V.; Hall, D. G. Catalytic Enantioselective and Catalyst-Controlled Diastereofacial-Selective Additions of Allyl- and Crotylboronates to Aldehydes Using Chiral Brønsted Acids. Angew. Chem. Int. Ed. 2006, 45, 2426–2428; (d) Zhang, P.; Morken, J. P. Catalytic Enantioselective Allylation of Dienals Through the Intermediacy of Unsaturated π-Allyl Complexes. J. Am. Chem. Soc. 2009, 131, 12550–12551; (e) Jain, P.; Antilla, J. C. Chiral Brønsted Acid-Catalyzed Allylboration of Aldehydes. J. Am. Chem. Soc. 2010, 132, 11884–11886; (f) Xing, C.-H.; Liao, Y.-X.; Zhang, Y.; Sabarova, D.; Bassous, M.; Hu, Q.-S. Asymmetric Allylboration of Aldehydes with Pinacol Allylboronates Catalyzed by 1,1′-Spirobiindane-7,7′-diol (SPINOL) Based Phosphoric Acids. Eur. J. Org. Chem. 2012, 1115–1118; (g) Miura, T.; Nishida, Y.; Morimoto, M.; Murakami, M. Enantioselective Synthesis of Anti Homoallylic Alcohols from Terminal Alkynes and Aldehydes Based on Concomitant Use of a Cationic Iridium Complex and a Chiral Phosphoric Acid. J. Am. Chem. Soc. 2013, 135, 11497–11500; (h) Incerti-Pradillos, C. A.; Kabeshov, M. A.; Malkov, A. V. Highly Stereoselective Synthesis of Z-Homoallylic Alcohols by Kinetic Resolution of Racemic Secondary Allyl Boronates. Angew. Chem. Int. Ed. 2013, 52, 5338–5341; (i) Gao, S.; Duan, M.; Andreola, L. R.; Yu, P.; Wheeler, S. E.; Houk, K. N.; Chen, M. Unusual Enantiodivergence in Chiral Brønsted Acid-Catalyzed Asymmetric Allylation with β-Alkenyl Allylic Boronates. Angew. Chem. Int. Ed. 2022, 61, e202208908.
- 12For selected asymmetric allylation of ketones, see: (a) Wada, R.; Oisaki, K.; Kanai, M.; Shibasaki, M. Catalytic Enantioselective Allylboration of Ketones. J. Am. Chem. Soc. 2004, 126, 8910–8911; (b) Lou, S.; Moquist, P. N.; Schaus, S. E. Asymmetric Allylboration of Ketones Catalyzed by Chiral Diols. J. Am. Chem. Soc. 2006, 128, 12660–12661; (c) Schneider, U.; Ueno, M. Catalytic Use of Indium(0) for Carbon− Carbon Bond Transformations in Water: General Catalytic Allylations of Ketones with Allylboronates. J. Am. Chem. Soc. 2008, 130, 13824–13825; (d) Shi, S.-L.; Xu, L.-W.; Oisaki, K.; Kanai, M.; Shibasaki, M. Identification of Modular Chiral Bisphosphines Effective for Cu(I)-Catalyzed Asymmetric Allylation and Propargylation of Ketones. J. Am. Chem. Soc. 2010, 132, 6638–6639; (e) Cui, Y.; Yamashita, Y.; Kobayashi, S. Facile Preparation of Allylzinc Species from Allylboronates and Zinc Amide via a Boron-to-Zinc Exchange Process and Their Reactions with Carbonyl Compounds, Imines and Hydrazones. Chem. Commun. 2012, 48, 10319–10321; (f) Zhang, Y.; Li, N.; Qu, B.; Ma, S.; Lee, H.; Gonnella, N. C.; Gao, J.; Li, W.; Tan, Z.; Reeves, J. T.; Wang, J.; Lorenz, J. C.; Li, G.; Reeves, D. C.; Pesmasiri, A.; Grinberg, N.; Haddad, N.; Lu, B. Z.; Song, J. J.; Senanayake, C. H. Asymmetric Methallylation of Ketones Catalyzed by a Highly Active Organocatalyst 3,3′-F2-BINOL. Org. Lett. 2013, 15, 1710–1713.
- 13For selected asymmetric allylation of ketimines, see: (a) Wada, R.; Shibuguchi, T.; Makino, S.; Oisaki, K.; Kanai, M.; Shibasaki, M. Catalytic Enantioselective Allylation of Ketoimines. J. Am. Chem. Soc. 2006, 128, 7687–7691; (b) Lou, S.; Moquist, P. N.; Schaus, S. E. Asymmetric Allylboration of Acyl Imines Catalyzed by Chiral Diols. J. Am. Chem. Soc. 2007, 129, 15398–15404; (c) Chakrabarti, A.; Konishi, H.; Yamaguchi, M.; Schneider, U.; Kobayashi, S. Indium(I)-Catalyzed Asymmetric Allylation, Crotylation, and α-Chloroallylation of Hydrazones with Rare Constitutional and High Configurational Selectivities. Angew. Chem. Int. Ed. 2010, 49, 1838–1841; (d) Luo, Y.; Hepburn, H. B.; Chotsaeng, N.; Lam, H. W. Enantioselective Rhodium-Catalyzed Nucleophilic Allylation of Cyclic Imines with Allylboron Reagents. Angew. Chem. Int. Ed. 2012, 51, 8309–8313; (e) Silverio, D. L.; Torker, S.; Pilyugina, T.; Vieira, E. M.; Snapper, M. L.; Haeffner, F.; Hoveyda, A. H. Simple Organic Molecules as Catalysts for Enantioselective Synthesis of Amines and Alcohols. Nature 2013, 494, 216–221.
- 14(a) Wang, J.; Zhang, Q.-X.; Zhou, B.-Y.; Yang, C.; Li, X.; Cheng, J.-P. Bi(III)-Catalyzed Enantioselective Allylation Reactions of Ketimines. iScience 2019, 16, 511–523; (b) Pan, Y.-L.; Zheng, H.-L.; Wang, J.; Yang, C.; Li, X.; Cheng, J.-P. Enantioselective Allylation of Oxocarbenium Ions Catalyzed by Bi(OAc)3/Chiral Phosphoric Acid. ACS Catal. 2020, 10, 8069–8076; (c) Wang, J.; Zhang, Q.; Li, Y.; Liu, X.; Li, X.; Cheng, J.-P. Bi(OAc)3/Chiral Phosphoric Acid Catalyzed Enantioselective Allylation of Isatins. Chem. Commun. 2020, 56, 261–264; (d) Cai, L.; Pan, Y.-L.; Chen, L.; Cheng, J.-P.; Li, X. Bi(OAc)3/Chiral Phosphoric Acid Catalyzed Enantioselective Allylation of Seven-Membered Cyclic Imines, Dibenzo[b,f][1,4]oxazepines. Chem. Commun. 2020, 56, 12383–12386; (e) Liu, X.-S.; Li, Y.; Li, X. Bi(OAc)3/Chiral Phosphoric Acid-Catalyzed Enantioselective 1,2- and Formal 1,4-Allylation Reaction of β,γ-Unsaturated α-Ketoesters. Org. Lett. 2021, 23, 9128–9133; (f) Pan, Y.-L.; Shao, Y.-B.; Wang, J.; Liu, Z.; Chen, L.; Li, X. Kinetic Resolution of 2H-Azirines by Asymmetric Allylation Reactions. ACS Catal. 2021, 11, 13752–13760.