Nickel-Catalyzed Asymmetric Reductive 1,4- and 1,5-Dicarbofunctionalization†
Yutong Xiang
Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorCorresponding Author
Chang Zhang
School of Materials Science and Engineering, Hefei Institute of Technology, Hefei, Anhui, 238076 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chuan Wang
Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
E-mail: [email protected]; [email protected]Search for more papers by this authorYutong Xiang
Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorCorresponding Author
Chang Zhang
School of Materials Science and Engineering, Hefei Institute of Technology, Hefei, Anhui, 238076 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chuan Wang
Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
E-mail: [email protected]; [email protected]Search for more papers by this author† Dedicated to the Special Issue of Catalytic Alkene Functionalization.
Comprehensive Summary
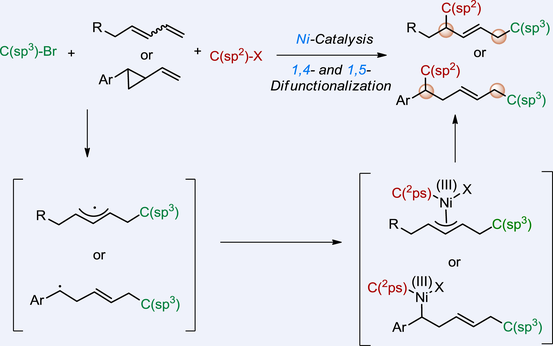
Herein, we present the first examples of asymmetric reductive 1,4-dicarbofunctionalization of 1,3-dienes and 1,5-dicarbofunctionalization of vinylcyclopropanes, which proceed under the catalysis of a chiral nickel/bis-imidazoline complex using alkyl halides and aryl iodides or alkenyl bromides as the electrophilic coupling partners. In these highly enantioselective transformations operating in a radical relay mechanism, the C(sp3)- and C(sp2)-type carbo-moieties are respectively installed on the terminal and internal position with a newly formed olefinic unit in high E-selectivity.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500074-sup-0001-supinfo.pdfPDF document, 24.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on olefin dicarbofunctionalization, see: (a) Han, J.; He, R.; Wang, C. Transition Metal-Catalyzed Asymmetric Three-Component Dicarbofunctionalization of Unactivated Alkenes. Chem. Catal. 2023, 3, 100690; (b) Dong, Z.; Song, L.; Chen, L.-A. Enantioselective Ni-Catalyzed Three-Component Dicarbofunctionalization of Alkenes. ChemCatChem 2023, 15, e202300803; (c) Kang, T.; Apolinar, O.; Engle, K. M. Ni- and Pd-Catalyzed Enantioselective 1,2-Dicarbofunctionalization of Alkenes. Synthesis 2023, 56, 1–15; (d) Zhu, S.; Zhao, X.; Li, H.; Chu, L. Catalytic Three-Component Dicarbofunctionalization Reactions Involving Radical Capture by Nickel. Chem. Soc. Rev. 2021, 50, 10836−10856; (e) Ping, Y.; Kong, W. Ni-Catalyzed Reductive Difunctionalization of Alkenes. Synthesis 2020, 52, 979–992; (f) Derosa, J.; Apolinar, O.; Kang, T.; Tran, V. T.; Engle, K. M. Recent Developments in Nickel-Catalyzed Intermolecular Dicarbofunctionalization of Alkenes. Chem. Sci. 2020, 11, 4287−4296; (g) Tu, H.-Y.; Zhu, S.; Qing, F.-L.; Chu, L. Recent Advances in Nickel-Catalyzed Three-Component Difunctionalization of Unactivated Alkenes. Synthesis 2020, 52, 1346−1356; (h) Qi, X.; Diao, T. Nickel-Catalyzed Dicarbofunctionalization of Alkenes. ACS Catal. 2020, 10, 8542−8556; (i) Luo, Y.-C.; Xu, C.; Zhang, X. Nickel-Catalyzed Dicarbofunctionalization of Alkenes. Chin. J. Chem. 2020, 38, 1371–1394; (j) Giri, R.; KC, S. Strategies toward Dicarbofunctionalization of Unactivated Olefins by Combined Heck Carbometalation and Cross-Coupling. J. Org. Chem. 2018, 83, 3013−3022.
- 2For selected examples of asymmetric redox-neutral olefin dicarbofunctionalization, see: (a) Wang, Z.-C.; Luo, X.; Zhang, J.-W.; Liu, C.-F.; Koh, M. J.; Shi, S.-L. Enantioselective C–C Cross-Coupling of Unactivated Alkenes. Nat. Catal. 2023, 6, 1087−1097; (b) Liu, C.-F.; Wang, Z.-C.; Luo, X.; Lu, J.; Ko, C. H. M.; Shi, S.-L.; Koh, M. J. Synthesis of Tri- and Tetrasubstituted Stereocentres by Nickel-Catalysed Enantioselective Olefin Cross-Couplings. Nat. Catal. 2022, 5, 934−942; (c) Apolinar, O.; Kang, T.; Alturaifi, T. M.; Bedekar, P. G.; Rubel, C. Z.; Derosa, J.; Sanchez, B. B.; Wong, Q. N.; Sturgell, E. J.; Chen, J. S.; Wisniewski, S. R.; Liu, P.; Engle, K. M. Three-Component Asymmetric Ni-Catalyzed 1,2-Dicarbofunctionalization of Unactivated Alkenes via Stereoselective Migratory Insertion. J. Am. Chem. Soc. 2022, 144, 19337−19343; (d) Xi, Y.; Huang, W.; Wang, C.; Ding, H.; Xia, T.; Wu, L.; Fang, K.; Qu, J.; Chen, Y. Catalytic Asymmetric Diarylation of Internal Acyclic Styrenes and Enamides. J. Am. Chem. Soc. 2022, 144, 8389−8398; (e) Guo, L.; Yuan, M.; Zhang, Y.; Wang, F.; Zhu, S.; Gutierrez, O.; Chu, L. General Method for Enantioselective Three-Component Carboarylation of Alkenes Enabled by Visible-Light Dual Photoredox/Nickel Catalysis. J. Am. Chem. Soc. 2020, 142, 20390−20399; (f) Fan, P.; Lan, Y.; Zhang, C.; Wang, C. Nickel/Photo-Cocatalyzed Asymmetric Acyl- Carbamoylation of Alkenes. J. Am. Chem. Soc. 2020, 142, 2180−2186; (g) Zhang, Z.-M.; Xu, B.; Wu, L.; Wu, Y.; Qian, Y.; Zhou, L.; Liu, Y.; Zhang, J. Enantioselective Dicarbofunctionalization of Unactivated Alkenes by Palladium-Catalyzed Tandem Heck/Suzuki Coupling Reaction. Angew. Chem. Int. Ed. 2019, 58, 14653−14659; (h) You, W.; Brown, M. K. Catalytic Enantioselective Diarylation of Alkenes. J. Am. Chem. Soc. 2015, 137, 14578−14581; (i) Cong, H.; Fu, G. C. Catalytic Enantioselective Cyclization/Cross-Coupling with Alkyl Electrophiles. J. Am. Chem. Soc. 2014, 136, 3788−3791.
- 3For reviews on reductive cross-electrophile coupling, see: (a) Everson, D. A.; Weix, D. J. Cross-Electrophile Coupling: Principles of Reactivity and Selectivity. J. Org. Chem. 2014, 79, 4793−4798;
(b) Moragas, T.; Correa, A.; Martin, R. Metal-Catalyzed Reductive Coupling Reactions of Organic Halides with Carbonyl-Type Compounds. Chem. Eur. J. 2014, 20, 8242−8258;
(c) Knappke, C. E. I.; Grupe, S.; Gärtner, D.; Corpet, M.; Gosmini, C.; von Wangelin, A. J. Reductive Cross-Coupling Reactions between Two Electrophiles. Chem. Eur. J. 2014, 20, 6828−6842;
(d) Gu, J.; Wang, X.; Xue, W.; Gong, H. Nickel-Catalyzed Reductive Coupling of Alkyl Halides with Other Electrophiles: Concept and Mechanistic Considerations. Org. Chem. Front. 2015, 2, 1411−1421;
(e) Weix, D. J. Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc. Chem. Res. 2015, 48, 1767−1775;
(f) Wang, X.; Dai, Y.; Gong, H. Nickel-Catalyzed Reductive Couplings. Top. Curr. Chem. 2016, 374, 43;
(g) Richmond, E.; Moran, J. Recent Advances in Nickel Catalysis Enabled by Stoichiometric Metallic Reducing Agents. Synthesis 2018, 50, 499−513;
(h) Poremba, K. E.; Dibrell, S. E.; Reisman, S. E. Nickel-Catalyzed Enantioselective Reductive Cross-Coupling Reactions. ACS Catal. 2020, 10, 8237−8246;
(i) Jin, Y.; Wang, C. Nickel-Catalyzed Asymmetric Cross-Electrophile Coupling Reactions. Synlett 2020, 31, 1843−1850;
(j) Yi, L.; Ji, T.; Chen, K.-Q.; Chen, X.-Y.; Rueping, M. Nickel-Catalyzed Reductive Cross-Couplings: New Opportunities for Carbon–Carbon Bond Formations through Photochemistry and Electrochemistry. CCS Chem. 2022, 4, 9−30;
(k) Pang, X.; Su, P.-F.; Shu, X.-Z. Reductive Cross-Coupling of Unreactive Electrophiles. Acc. Chem. Res. 2022, 55, 2491−2509;
(l) Pan, Q.; Ping, Y.; Kong, W. Nickel-Catalyzed Ligand-Controlled Selective Reductive Cyclization/Cross-Couplings. Acc. Chem. Res. 2023, 56, 515–535;
(m) Ehehalt, L. E.; Beleh, O. M.; Priest, I. C.; Mouat, J. M.; Olszewski, A. K.; Ahern, B. N.; Cruz, A. R.; Chi, B. K.; Castro, A. J.; Kang, K.; Wang, J.; Weix, D. J. Cross-Electrophile Coupling: Principles, Methods, and Applications in Synthesis. Chem. Rev. 2024, 104, 13397–13569.
10.1021/acs.chemrev.4c00524 Google Scholar
- 4(a) Wang, K.; Ding, Z.; Zhou, Z.; Kong, W. Ni-Catalyzed Enantioselective Reductive Diarylation of Activated Alkenes by Domino Cyclization/Cross-Coupling. J. Am. Chem. Soc. 2018, 140, 12364−12368; (b) Jin, Y.; Wang, C. Nickel-Catalyzed Asymmetric Reductive Arylalkylation of Unactivated Alkenes. Angew. Chem. Int. Ed. 2019, 58, 6722−6726; (c) Tian, Z.-X.; Qiao, J.-B.; Xu, G.-L.; Pang, X.; Qi, L.; Ma, W.-Y.; Zhao, Z.-Z. Duan, J.; Du, Y.-F.; Su, P.; Liu, X.-Y.; Shu, X.-Z. Highly Enantioselective Cross-Electrophile Aryl-Alkenylation of Unactivated Alkenes. J. Am. Chem. Soc. 2019, 141, 7637−7643; (d) Lan, Y.; Wang, C. Nickel-catalyzed Enantioselective Reductive Carbo-Acylation of Alkenes. Commun. Chem. 2020, 3, 45; (e) Wu, X.; Qu, J.; Chen, Y. Quinim: A New Ligand Scaffold Enables Nickel-Catalyzed Enantioselective Synthesis of α-Alkylated γ-Lactam. J. Am. Chem. Soc. 2020, 142, 15654−15660; (f) Pan, Q.; Ping, Y.; Wang, Y.; Guo, Y.; Kong, W. Ni-Catalyzed Ligand-Controlled Regiodivergent Reductive Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc. 2021, 143, 10282−10291; (g) Qiao, J.-B.; Zhang, Y.-Q.; Yao, Q.-W.; Zhao, Z.-Z.; Peng, X.; Shu. X.-Z. Enantioselective Reductive Divinylation of Unactivated Alkenes by Nickel-Catalyzed Cyclization Coupling Reaction. J. Am. Chem. Soc. 2021, 143, 12961; (h) Zhao, T.-Y.; Xiao, L.-J.; Zhou, Q.-L. Nickel-Catalyzed Desymmetric Reductive Cyclization/Coupling of 1,6-Dienes: An Enantioselective Approach to Chiral Tertiary Alcohol. Angew. Chem. Int. Ed. 2022, 61, e202115702; (i) Ma, Z.; Xu, W.; Wu, Y.-D.; Zhou, J.-S. Cobalt-Catalyzed Enantioselective Cross-Electrophile Couplings: Stereoselective Syntheses of 5–7-Membered Azacycles. J. Am. Chem. Soc. 2023, 145, 16464−16473; (j) Ding, D.; Zhang, L.; Wen, H.; Wang, C. Cobalt-Catalyzed Asymmetric Reductive Dicarbofunctionalization of 1,3-Dienes with o-Bromoaryl Imines as a Bis-Electrophile. ACS Catal. 2023, 13, 744–748.
- 5(a) Anthony, D.; Lin, Q.; Baudet, J.; Diao, T. Nickel-Catalyzed Asymmetric Reductive Diarylation of Vinylarenes. Angew. Chem. Int. Ed. 2019, 58, 3198−3202; (b) Dong, Z.; Tang, Q.; Xu, C.; Chen, L.; Ji, H.; Zhou, S.; Song, L.; Chen, L.-A. Directed Asymmetric Nickel-Catalyzed Reductive 1,2-Diarylation of Electronically Unactivated Alkenes. Angew. Chem. Int. Ed. 2023, 62, e202218286.
- 6 Wei, X.; Shu, W.; García-Domínguez, A.; Merino, E.; Nevado, C. Asymmetric Ni-Catalyzed Radical Relayed Reductive Coupling. J. Am. Chem. Soc. 2020, 142, 13515−13522.
- 7(a) Tu, H.-Y.; Wang, F.; Huo, L.-P.; Li, Y.; Zhu, S.; Zhao, X.; Li, H.; Qing, F.-L.; Chu, L. Enantioselective Three-Component Fluoroalkylarylation of Unactivated Olefins through Nickel-Catalyzed Cross-Electrophile Coupling. J. Am. Chem. Soc. 2020, 142, 9604−9611; (b) Wang, F.; Pan, S.; Zhu, S.; Chu, L. Selective Three-Component Reductive Alkylalkenylation of Unbiased Alkenes via Carbonyl-Directed Nickel Catalysis. ACS Catal. 2022, 12, 9779−9789.
- 8(a) Qian, P.; Guan, H.; Wang, Y.-E.; Lu, Q.; Zhang, F.; Xiong, D.; Walsh, P. J.; Mao, J. Catalytic Enantioselective Reductive Domino Alkyl Arylation of Acrylates via Nickel/Photoredox Catalysis. Nat. Commun. 2021, 12, 6613; (b) Gao, X.; Lin, T.; Wang, Y.-E.; Xing, F.; Qiu, Y.; Xiong, D.; Mao, J. Nickel/Photoredox-Catalyzed Asymmetric Three-Component Cross-Coupling to Access Enantioenriched 1,1-Diaryl(heteroaryl)alkanes. Org. Lett. 2024, 26, 8792−8797.
- 9 Wang, Y.-Z.; Sun, B.; Zhu, X.-Y.; Gu, Y.-C.; Ma, C.; Mei, T.-S. Enantioselective Reductive Cross-Couplings of Olefins by Merging Electrochemistry with Nickel Catalysis. J. Am. Chem. Soc. 2023, 145, 23910−23917.
- 10 Li, Y.-Z.; Rao, N.; An, L.; Wan, X.-L.; Zhang, Y.; Zhang, X. Enantioselective Nickel-Catalyzed Dicarbofunctionalization of 3,3,3-Trifluoropropene. Nat. Commun. 2022, 13, 5539.
- 11 Dong, Z.; Xu, C.; Chang, J.; Zhou, S.; Sun, P.; Li, Y.; Chen, L.-A. Enantioselective Directed Nickel-Catalyzed Three-Component Reductive Arylalkylation of Alkenes via the Carbometalation/Radical Cross- Coupling Sequence. ACS Catal. 2024, 14, 4395−4406.
- 12 Xiao, J.; Jia, T.; Chen, S.; Pan, M.; Li, X. Ni-Catalyzed Enantioselective Three-Component Reductive Alkylacylation of Alkenes: Modular Access to Structurally Complex α-Amino Ketones. Chem. Sci. 2024, 15, 15489−15495.
- 13For reviews on asymmetric difunctionalization of 1,3-dienes, see: (a) Xiong, Y.; Sun, Y.; Zhang, G. Recent Advances on Catalytic Asymmetric Difunctionalization of 1,3-Dienes. Tetrahedron Lett. 2018, 59, 347−355; (b) Wu, X.; Gong, L.-Z. Palladium(0)-Catalyzed Difunctionalization of 1,3-Dienes: From Racemic to Enantioselective. Synthesis 2019, 51, 122−134; (c) Li, G.; Huo, X.; Jiang, X.; Zhang, W. Asymmetric Synthesis of Allylic Compounds via Hydrofunctionalisation and Difunctionalisation of Dienes, Allenes, and Alkynes. Chem. Soc. Rev. 2020, 49, 2060−2118; (d) Perry, G. J.; Jia, T.; Procter, D. J. Copper- Catalyzed Functionalization of 1,3-Dienes: Hydrofunctionalization, Borofunctionalization, and Difunctionalization. ACS Catal. 2020, 10, 1485−1499.
- 14(a) Stokes, B. J.; Liao, L.; de Andrade, A. M.; Wang, Q.; Sigman, M. S. A Palladium-Catalyzed Three-Component-Coupling Strategy for the Differential Vicinal Diarylation of Terminal 1,3-Dienes. Org. Lett. 2014, 16, 4666−4669; (b) Wu, X.; Lin, H.-C.; Li, M.-L.; Li, L.-L.; Han, Z.-Y.; Gong, L.-Z. Enantioselective 1,2-Difunctionalization of Dienes Enabled by Chiral Palladium Complex-Catalyzed Cascade Arylation/Allylic Alkylation Reaction. J. Am. Chem. Soc. 2015, 137, 13476−13479; (c) Cooze, C. J. C.; McNutt, W.; Schoetz, M. D.; Sosunovych, B.; Grigoryan, S.; Lundgren, R. J. Diastereo-, Enantio-, and Z-Selective α,δ-Difunctionalization of Electron-Deficient Dienes Initiated by Rh-Catalyzed Conjugate Addition. J. Am. Chem. Soc. 2021, 143, 10770–10777; (d) Marcum, J. S.; Meek, S.-J. Efficient Enantio-, Diastereo, E/Z-, and Site-Selective Nickel-Catalyzed Fragment Couplings of Aldehydes, Dienes, and Organoborons. J. Am. Chem. Soc. 2022, 144, 19231−19231; (e) Yu, H.; Zhang, Q.; Zi, W. Enantioselective Three-Component Photochemical 1,4-Bisalkylation of 1,3-Butadiene with Pd/Cu Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202208411.
- 15(a) Liu, Y.; Xie, Y.; Wang, H.; Huang, H. Enantioselective Aminomethylamination of Conjugated Dienes with Aminals Enabled by Chiral Palladium Complex-Catalyzed C–N Bond Activation. J. Am. Chem. Soc. 2016, 138, 4314−4317; (b) Chen, S.-S.; Wu, M.-S.; Han, Z.-Y. Palladium-Catalyzed Cascade sp2 C−H Functionalization/Intramolecular Asymmetric Allylation: From Aryl Ureas and 1,3-Dienes to Chiral Indolines. Angew. Chem. Int. Ed. 2017, 56, 6641−6645.
- 16(a) Chang, R.; Cai, S.; Yang, G.; Yan, X.; Huang, H. Asymmetric Aminomethylative Etherification of Conjugated Dienes with Aliphatic Alcohols Facilitated by Hydrogen Bonding. J. Am. Chem. Soc. 2021, 143, 12467−12472; (b) Chen, J.; Liang, Y.-J.; Wang, P.-Z.; Li, G.-Q.; Zhang, B.; Qian, H.; X.-D. Huan, Guan, W.; Xiao, W.-J.; Chen, J.-R. Photoinduced Copper-Catalyzed Asymmetric C–O Cross-Coupling. J. Am. Chem. Soc. 2021, 143, 13382−13392; (c) Tu, Y.; Xu, B.; Wang, Q.; Dong, H.; Zhang, Z.-M.; Zhang, J. Palladium/TY-Phos-Catalyzed Asymmetric Heck/Tsuji–Trost Reaction of o-Bromophenols with 1,3-Dienes. J. Am. Chem. Soc. 2023, 145, 4378−4383.
- 17 Du, H.; Yuan, W.; Zhao, B.; Shi, Y. Catalytic Asymmetric Diamination of Conjugated Dienes and Triene. J. Am. Chem. Soc. 2007, 129, 11688−11689.
- 18(a) Williamson, K. S.; Yoon, T. P. Iron Catalyzed Asymmetric Oxyamination of Olefins. J. Am. Chem. Soc. 2012, 134, 12370−12373; (b) Shen, H.-C.; Wu, Y.-F.; Zhang, Y.; Fan, L.-F.; Han, Z.-Y.; Gong, L.-Z. Palladium-Catalyzed Asymmetric Aminohydroxylation of 1,3-Dienes. Angew. Chem. Int. Ed. 2018, 57, 2372−2376.
- 19(a) Jiang, L.; Cao, P.; Wang, M.; Chen, B.; Wang, B.; Liao, J. Highly Diastereo- and Enantioselective Cu-Catalyzed Borylative Coupling of 1,3-Dienes and Aldimines. Angew. Chem. Int. Ed. 2016, 55, 13854–13858; (b) Feng, J.-J.; Oestreich, M. Tertiary α-Silyl Alcohols by Diastereoselective Coupling of 1,3-Dienes and Acylsilanes Initiated by Enantioselective Copper-Catalyzed Borylation. Angew. Chem. Int. Ed. 2019, 58, 8211–8215; (c) Sardini, S. R.; Brown, M. K. Catalyst Controlled Regiodivergent Arylboration of Dienes. J. Am. Chem. Soc. 2017, 139, 9823−9826; (d) Mao, J.-T.; Zhang, T.; Yao, B.-Y.; Xiao, L.-J.; Zhou, Q.-L. Diastereodivergent and Enantioselective Synthesis of Homoallylic Alcohols via Nickel-Catalyzed Borylative Coupling of 1,3-Dienes with Aldehydes. J. Am. Chem. Soc. 2023, 145, 19195−19201.
- 20(a) Burks, S. E.; Kliman, L. T.; Morken, J. P. Asymmetric 1,4-Dihydroxylation of 1,3-Dienes by Catalytic Enantioselective Diboration. J. Am. Chem. Soc. 2009, 131, 9134−9135; (b) Kliman, L. T.; Mlynarski, L. N.; Ferris, G. E.; Morken, J. P. Catalytic Enantioselective 1,2-Diboration of 1,3-Dienes: Versatile Reagents for Stereoselective Allylation. Angew. Chem. Int. Ed. 2012, 51, 521−524.
- 21 Xiong, Y.; Zhang, G. Enantioselective 1,2-Difunctionalization of 1,3-Butadiene by Sequential Alkylation and Carbonyl Allylation. J. Am. Chem. Soc. 2018, 140, 2735−2738.
- 22 Zhang, W.-S.; Ji, D.-W.; Li, Y.; Zhang, X.-X.; Zhao, C.-Y.; Hu, Y.-C.; Chen, Q.-A. Regio- and Stereoselective Diarylation of 1,3-Dienes via Ni/Cr Cocatalysis. ACS Catal. 2022, 12, 2158–2165.
- 23For reviews on asymmetric ring opening of vinyl cyclopropanes, see: (a) Pirenne, W.; Muriel, B.; Waser, J. Catalytic Enantioselective Ring-Opening Reactions of Cyclopropanes. Chem. Soc. Rev. 2021, 121, 227–263; (b) Adhikari, S. A.; Majumdar, N. Catalytic Asymmetric Ring Opening Reactions of Vinylcyclopropanes. Eur. J. Org. Chem. 2024, 27, e202301225.
- 24(a) Chen, C.; Shen, X.; Chen, J.; Hong, X.; Lu, Z. Iron-Catalyzed Hydroboration of Vinylcyclopropanes. Org. Lett. 2017, 19, 5422–5425; (b) Zhang, Z.-Q.; Meng, X.-Y.; Sheng, J.; Lan, Q.; Wang, X.-S. Enantioselective Copper-Catalyzed 1,5-Cyanotrifluoromethylation of Vinylcyclopropanes. Org. Lett. 2019, 21, 8256–8260; (c) Chen, C.; Wang, H.; Sun, Y.; Cui, J.; Xie, J.; Shi, Y.; Yu, S.; Hong, X.; Lu, Z. Iron-Catalyzed Asymmetric Hydrosilylation of Vinylcyclopropanes via Stereospecific C-C Bond Cleavage. iScience 2020, 23, 100985.
- 25For summary of the unsuccessful substrates, see: SI, Page S38.