Pd(II)-Catalyzed Selective [4+2] Benzannulations of Pyridones with Alkenes: Diversity-Oriented Synthesis of a Novel Fluorescent Quinolinone
Yiwei Xu
Key Laboratory of Xin′an Medicine, Ministry of Education, Anhui University of Chinese Medicine, Hefei, Anhui, 230038 China
Search for more papers by this authorYuanyuan Wang
Key Laboratory of Xin′an Medicine, Ministry of Education, Anhui University of Chinese Medicine, Hefei, Anhui, 230038 China
Search for more papers by this authorJing Li
Key Laboratory of Xin′an Medicine, Ministry of Education, Anhui University of Chinese Medicine, Hefei, Anhui, 230038 China
Search for more papers by this authorJinxiang Ye
Key Laboratory of Xin′an Medicine, Ministry of Education, Anhui University of Chinese Medicine, Hefei, Anhui, 230038 China
Search for more papers by this authorCorresponding Author
Hui Miao
Anhui Province Key Laboratory of Environmental Hormone and Reproduction, School of Biological and Food Engineering, Fuyang Normal University, Fuyang, Anhui, 236037 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Qianwen Gao
College of Chemistry and Chemical Engineering, State Key Laboratory of Chemo/Biosensing and Chemometrics, Advanced Catalytic Engineering Research Center of the Ministry of Education, Hunan University, Changsha, Hunan, 410082 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chenggui Wu
Key Laboratory of Xin′an Medicine, Ministry of Education, Anhui University of Chinese Medicine, Hefei, Anhui, 230038 China
Anhui Province Key Laboratory of Environmental Hormone and Reproduction, School of Biological and Food Engineering, Fuyang Normal University, Fuyang, Anhui, 236037 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorYiwei Xu
Key Laboratory of Xin′an Medicine, Ministry of Education, Anhui University of Chinese Medicine, Hefei, Anhui, 230038 China
Search for more papers by this authorYuanyuan Wang
Key Laboratory of Xin′an Medicine, Ministry of Education, Anhui University of Chinese Medicine, Hefei, Anhui, 230038 China
Search for more papers by this authorJing Li
Key Laboratory of Xin′an Medicine, Ministry of Education, Anhui University of Chinese Medicine, Hefei, Anhui, 230038 China
Search for more papers by this authorJinxiang Ye
Key Laboratory of Xin′an Medicine, Ministry of Education, Anhui University of Chinese Medicine, Hefei, Anhui, 230038 China
Search for more papers by this authorCorresponding Author
Hui Miao
Anhui Province Key Laboratory of Environmental Hormone and Reproduction, School of Biological and Food Engineering, Fuyang Normal University, Fuyang, Anhui, 236037 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Qianwen Gao
College of Chemistry and Chemical Engineering, State Key Laboratory of Chemo/Biosensing and Chemometrics, Advanced Catalytic Engineering Research Center of the Ministry of Education, Hunan University, Changsha, Hunan, 410082 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chenggui Wu
Key Laboratory of Xin′an Medicine, Ministry of Education, Anhui University of Chinese Medicine, Hefei, Anhui, 230038 China
Anhui Province Key Laboratory of Environmental Hormone and Reproduction, School of Biological and Food Engineering, Fuyang Normal University, Fuyang, Anhui, 236037 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
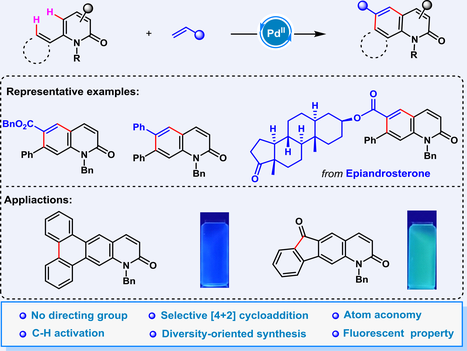
Herein, an intermolecular palladium(II)-catalyzed regioselective [4+2] benzannulation reaction capable of converting 2-pyridones into quinolinones was developed using electron-deficient alkenes as two-carbon units. An examination of the reaction mechanism indicated that the extension from 2-pyridone to quinolinone was likely facilitated through a series of sequential C—H activation reactions or 6π electrocyclization, culminating in dehydrogenative aromatization. This method of diversity-oriented synthesis of quinolinone derivatives is characterized by a broad substrate scope, atom economy, and excellent chemical selectivity. In addition, these quinolinone derivatives exhibit fluorescent absorption within the visible-light spectrum, which makes them suitable candidates for the development of innovative fluorescent probes.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500045-sup-0001-supinfo.pdfPDF document, 5.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Rao, L. B.; Sreenivasulu, C.; Kishore, D. R.; Satyanarayana, G. Trending strategies for the synthesis of quinolinones and isoquinolinones. Tetrahedron 2022, 127, 133093.
- 2(a) Beattie, D.; Beer, D.; Bradley, M. E.; Bruce, I.; Charlton, S. J.; Cuenoud, B. M.; Fairhurst, R. A.; Farr, D.; Fozard, J. R.; Janus, D.; Rosethorne, E. M.; Sandham, D. A.; Sykes, D. A.; Trifilieff, A.; Turner, K. L.; Wissler, E. An Investigation into the Structure–Activity Relationships Associated with the Systematic Modification of the β2-adrenoceptor Agonist Indacaterol. Bioorg. Med. Chem. Lett. 2012, 22, 6280–6285;
(b) Konecny, G. E.; Winterhoff, B.; Guorong, E. Y.; Qi, J.; Le, J.; Shi, M.; Dugan, M.; Linnartz, R.; Finn, R. S.; Slamon, D. J. Abstract 3589: Dovitinib (TKI258), A Multikinase Inhibitor of FGFR, PDGFR, and VEGFR Tyrosine Kinases, Induces Growth Inhibition in Endometrial Carcinoma cells. Cancer Res. 2011, 71, 3589–3589.
10.1158/1538-7445.AM2011-3589 Google Scholar
- 3(a) Kim, I.; Park, B.; Kang, G.; Kim, J.; Jung, H.; Lee, H.; Baik, M.; Hong, S. Visible-Light-Induced Pyridylation of Remote C(sp3)−H Bonds by Radical Translocation of N-Alkoxypyridinium Salts. Angew. Chem. Int. Ed. 2018, 57, 15517–15522; (b) Kim, I.; Kang, G.; Lee, K.; Park, B.; Kang, D.; Jung, H.; He, Y.-T.; Baik, M.-H.; Hong, S. Site-Selective Functionalization of Pyridinium Derivatives via Visible-Light-Driven Photocatalysis with Quinolinone. J. Am. Chem. Soc. 2019, 141, 9239–9248; (c) Vellakkaran, M.; Hong, S. Visible-light-induced Reactions Driven by Photochemical Activity of Quinolinone and Coumarin Scaffolds. Asian J. Org. Chem. 2021, 10, 1012–1023.
- 4 Li, Z.; Wang, Z.; Chekshin, N.; Qian, S.; Qiao, J. X.; Cheng, P. T.; Yeung, K.; Ewing, W. R.; Yu, J.-Q. A Tautomeric Ligand Enables Directed C–H Hydroxylation with Molecular Oxygen. Science 2021, 372, 1452–1457.
- 5For selected examples: (a) Liu, L.; Lu, H.; Wang, H.; Yang, C.; Zhang, X.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. PhI(OCOCF3)2-Mediated C–C Bond Formation Concomitant with a 1,2-Aryl Shift in a Metal-Free Synthesis of 3-Arylquinolin-2-ones. Org. Lett. 2013, 15, 2906–2909; (b) Deng, Y.; Gong, W.; He, J.; Yu, J.-Q. Ligand-Enabled Triple C-H Activation Reactions: One-Pot Synthesis of Diverse 4-Aryl-2-quinolinones from Propionamides. Angew. Chem. Int. Ed. 2014, 53, 6692–6695; (c) Zhang, D.; Gao, F.; Nian, Y.; Zhou, Y.; Jiang, H.; Liu, H. Palladium-Catalyzed Picolinamide-Directed Coupling of C(sp2)–H and C(sp2)–H: a Straightforward Approach to Quinolinone and Pyridone Scaffolds. Chem. Commun. 2015, 51, 7509–7511; (d) Wu, J.; Xiang, S.; Zeng, J.; Leow, M.; Liu, X.-W. Practical Route to 2-Quinolinones via a Pd-Catalyzed C–H Bond Activation/C–C Bond Formation/Cyclization Cascade Reaction. Org. Lett. 2015, 17, 222–225; (e) Zeng, R.; Dong, G. Rh-Catalyzed Decarbonylative Coupling with Alkynes via C–C Activation of Isatins. J. Am. Chem. Soc. 2015, 137, 1408–1411; (f) Zhang, Z.; Qian, J.; Zhang, G.; Ma, N.; Liu, Q.; Liu, T.; Sun, K.; Shi, L. Copper(I) Catalyzed C(sp2)–N bond Formation: Synthesis of Pyrrolo[3,2-c]quinolinone Derivatives. Org. Chem. Front. 2016, 3, 344–348; (g) Huang, B.; Shen, Y.; Mao, Z.; Liu, Y.; Cui, S. Metathesis Reaction of Diazo Compounds and para-Quinone Methides for C–C Double Bond Formation: Synthesis of Tetrasubstituted Alkenes and Quinolinones. Org. Lett. 2016, 18, 4888–4891; (h) Guan, M.; Pang, Y.; Zhang, J.; Zhao, Y. Pd-Catalyzed Sequential β-C(sp3)–H Arylation and Intramolecular Amination of δ-C(sp2)–H Bonds for Synthesis of Quinolinones via an N,O-bidentate Directing Group. Chem. Commun. 2016, 52, 7043–7046; (i) Xiao, H.-Z.; Wang, W.-S.; Sun, Y.-S.; Luo, H.; Li, B.-W.; Wang, X.-D.; Lin, W.-L.; Luo, F.-X. Pd/Cu-Catalyzed Cascade C(sp3)–H Arylation and Intramolecular C–N Coupling: A One-Pot Synthesis of 3,4-2H-Quinolinone Skeletons. Org. Lett. 2019, 21, 1668–1671; (j) Yokoo, K.; Mori, K. Expeditious Synthesis of Multisubstituted Quinolinone Derivatives Based on Ring Recombination Strategy. Org. Lett. 2020, 22, 244–248; (k) Zheng, Y.; Wang, Z.-W.; Cheng, W.-S.; Xie, Z.-Z.; He, X.-C.; Chen, Y.-S.; Chen, K.; Xiang, H.-Y.; Chen, X.-Q.; Yang, H. Phosphine-Mediated Morita–Baylis–Hillman-Type/Wittig Cascade: Access to E-Configured 3-Styryl- and 3-(Benzopyrrole/furan-2-yl) Quinolinones. J. Org. Chem. 2022, 87, 974–984.
- 6For selected examples: (a) Chen, M.; Rago, A. J.; Dong, G. Platinum- Catalyzed Desaturation of Lactams, Ketones, and Lactones. Angew. Chem. Int. Ed. 2018, 57, 16205–16209; (b) Jeong, H. J.; Chae, S.; Jeong, K.; Namgoong, S. K. The Diverse One-Pot Reactions of 2-Quinolylzincates: Homologation, Electrophilic Trapping, Hydroxylation, and Arylation Reactions. Eur. J. Org. Chem. 2018, 6343–6349; (c) Peng, J.-B.; Chen, B.; Qi, X.; Ying, J.; Wu, X.-F. Palladium-Catalyzed Synthesis of Quinolin-2(1H)-ones: the Unexpected Reactivity of Azodicarboxylate. Org. Biomol. Chem. 2018, 16, 1632–1635; (d) Jin, Y.; Ou, L.; Yang, H.; Fu, H. Visible-Light-Mediated Aerobic Oxidation of N-Alkylpyridinium Salts under Organic Photocatalysis. J. Am. Chem. Soc. 2017, 139, 14237–14243; (e) Mandal, S.; Bhuyan, S.; Jana, S.; Hossain, J.; Chhetria, K.; Gopal Roy, B. Efficient Visible Light Mediated Synthesis of Quinolin-2(1H)-ones from Quinoline N-Oxides. Green Chem. 2021, 23, 5049–5055.
- 7(a) Biswas, A.; Giri, D.; Das, D.; De, A.; Patra, S. K.; Samanta, R. A Mild Rhodium Catalyzed Direct Synthesis of Quinolones from Pyridones: Application in the Detection of Nitroaromatics. J. Org. Chem. 2017, 82, 10989−10996; (b) Zhou, C.-J.; Gao, H.; Huang, S.-L.; Zhang, S.-S.; Wu, J.-Q.; Li, B.; Jiang, X.; Wang, H. Synthesis of Benzofused N-Heterocycles via Rh(III)-Catalyzed Direct Benzannulation with 1,3-Dienes. ACS Catal. 2019, 9, 556−564; (c) Prusty, N.; Mohanty, S. R.; Banjare, S. K.; Nanda, T.; Ravikumar, P. C. Switching the Reactivity of the Nickel-Catalyzed Reaction of 2-Pyridones with Alkynes: Easy Access to Polyaryl/Polyalkyl Quinolinones. Org. Lett. 2022, 24, 6122−6127; (d) Yadava, S. K.; Jeganmohan, M. Co(III)-catalyzed Regioselective Benzannulation of Substituted Pyridones with 1,6-diynes via Dual C–H Bond Activation. Chem. Commun. 2024, 60, 8296−8299.
- 8 Sun, Z.; He, F.; Xu, Y.; Lu, M.; Xiong, H., Jiang, Z.; Wu, C. Intramolecular Palladium(II)-Catalyzed Regioselective 6-endo or 6-exo C–H Benzannulation: An Approach for the Diversity-Oriented Synthesis of Quinolinone Derivatives from Pyridones. J. Org. Chem. 2024, 89, 7058–7064.
- 9(a) Itahara, T.; Ouseto, F. Alkenylation of 1-Methyl-2-pyridone and 2-Formylfuran with Methyl Acrylate and Palladium Acetate. Synthesis 1984, 488–489; (b) Chen, Y.; Wang, F.; Jia, A.; Li, X. Palladium-Catalyzed Selective Oxidative Olefination and Arylation of 2-Pyridones. Chem. Sci. 2012, 3, 3231–3236; (c) Gigant, N.; Bäckvall, J.-E. Synthesis of Conjugated Dienes via a Biomimetic Aerobic Oxidative Coupling of Two Cvinyl-H Bonds. Chem.–Eur. J. 2013, 19, 10799–1080; (d) Prendergast, A. M.; Pardo, L. M.; Fairlamb, I. J. S.; McGlacken, G. P. Access to Some C5-Cyclised 2-Pyrones and 2-Pyridones via Direct Arylation; Retention of Chloride as a Synthetic Handle. Eur. J. Org. Chem. 2017, 5119–5124; (e) Maity, S.; Das, D.; Sarkar, S. Samanta, R. Direct Pd(II)-Catalyzed Site-Selective C5-Arylation of 2-Pyridone Using Aryl Iodides. Org. Lett. 2018, 20, 5167−5171; (f) Sun, Z.; Jiang, Z.; He, F.; Li, C.; Xiong, H.; Yang, D.; Miao, H.; Li Q., Ye, J.; Wu, C. Site-Selective C−H Arylation of 2-Pyridones via Pd/NBE Cooperative Catalysis. ACS Catal. 2024, 14, 7762−7770.
- 10(a) Ozaki, K.; Zhang, H.; Ito, H.; Lei, A. Itami, K. One-shot Indole-to-carbazole p-Extension by a Pd–Cu–Ag Trimetallic System. Chem. Sci. 2013, 4, 3416–3420; (b) Laha, J. K.; Dayal, N. A Tandem Approach to Functionalized Carbazoles from Indoles via Two Successive Regioselective Oxidative Heck Reactions Followed by Thermal Electrocyclization. Org. Lett. 2015, 17, 3658–3661; (c) Saunthwal, R. K.; Patel, M.; Kumar, S.; Danodia, A. K.; Verma, A. K. Pd(II)-Catalyzed C–H Activation of Styrylindoles: Short, Efficient, and Regioselective Synthesis of Functionalized Carbazoles. Chem. Eur. J. 2015, 21, 18601–18605; (d) Zhang, D.; Xu, G.; Ding, D.; Zhu, C.; Li, J.; Sun, J. Gold(I)-Catalyzed Diazo Coupling: Strategy towards Alkene Formation and Tandem Benzannulation. Angew. Chem. Int. Ed. 2014, 53, 11070–11074; (e) Wu, J.; Yang, Z.; Zhang, S.; Jiang, C.; Li, Q.; Huang, Z.; Wang, H. From Indoles to Carbazoles: Tandem Cp*Rh(III)-Catalyzed C–H Activation/Brønsted Acid-Catalyzed Cyclization Reactions. ACS Catal. 2015, 5, 6453–6457.
- 11 Moritomo, A.; Yamada, H.; Watanabe, T.; Itahana, H.; Koga, Y.; Akuzawa, S.; Okada, M. Synthesis and Structure–activity Relationships of New Carbonyl guanidine Derivatives as Novel Dual 5-HT2B and 5-HT7 Receptor Antagonists. Part 2. Bioorg. Med. Chem. 2014, 22, 4323–4337.
- 12 Iwasaki, M.; Araki, Y.; Iino, S.; Nishihara, Y. Synthesis of Multisubstituted Triphenylenes and Phenanthrenes by Cascade Reaction of o-Iodobiphenyls or (Z)-β-Halostyrenes with o-Bromobenzyl Alcohols through Two Sequential C−C Bond Formations Catalyzed by a Palladium Complex. J. Org. Chem. 2015, 80, 9247–9263.
- 13 Zhang, C.; Liu, C.; Nie, S.; Li, X.; Wang, Y.; Zhang, Y.; Guo, J.; Sun, Y. Two Novel Fluorescent Probes Based on Quinolinone for Continuous Recognition of Al3+ and ClO−. Spectrochim. Acta A 2023, 300, 122917.