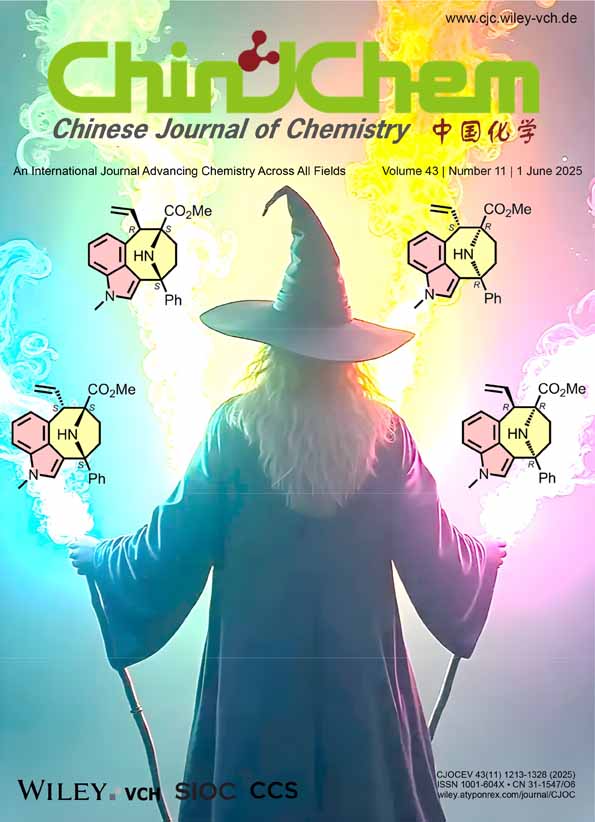
Cover Picture
Abstract
One-pot sequential reaction combining bimetallic Cu/Ir-catalyzed asymmetric allylation of ketimine esters and (E)-4-indolyl allyl carbonates with acid-mediated Pictet-Spengler cyclization was successfully developed. This protocol enables stereodivergent access to four stereoisomers of indole-fused 9-azabicyclo[4.2.1]nonanes, containing an eight-membered ring system bearing one tertiary and two quaternary stereocenters. It features a remarkable step economy, broad substrate compatibility, and excellent stereoselective control. More details are discussed in the article by Wang et al. on pages 1223—1229.