Dual Cu/Ir Catalyzed Asymmetric Allylation and Pictet-Spengler Cyclization: Stereodivergent Access to Chiral Indole Fused 9-Azabicyclo[4.2.1]nonanes†
Xin-Lian Liu
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorLu Xiao
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorYi Liu
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorYan Li
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorZuo-Fei Wang
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorXin Chang
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Xiu-Qin Dong
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chun-Jiang Wang
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 230021 China
E-mail: [email protected]; [email protected]Search for more papers by this authorXin-Lian Liu
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorLu Xiao
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorYi Liu
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorYan Li
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorZuo-Fei Wang
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorXin Chang
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Xiu-Qin Dong
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chun-Jiang Wang
Hubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 230021 China
E-mail: [email protected]; [email protected]Search for more papers by this author† Dedicated to the Special Issue of Catalytic Alkene Functionalization.
Comprehensive Summary
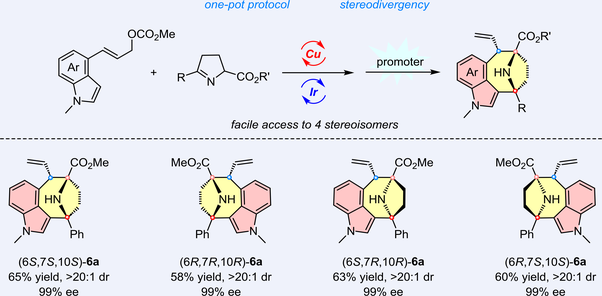
Nitrogen-containing bridged-heterocycles and indoles are key subunits of many natural products and pharmacologically active molecules. We herein present a bimetallic Cu/Ir catalyzed asymmetric allylation of ketimine esters and (E)-4-indolyl allyl carbonates followed by acid-promoted Pictet-Spengler cyclization sequences, enabling stereodivergent synthesis of chiral indole fused 9-azabicyclo[4.2.1]nonanes containing an eight-membered ring with one tertiary and two quaternary stereogenic centers. This one-pot sequential protocol features step economy, good substrate tolerance, and excellent stereoselective control.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500016-sup-0001-Supinfo.pdfPDF document, 7.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Vitaku, E.; Smith, D. T. J.; Njardarson, T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274.
- 2 Bhutani, P.; Joshi, G.; Raja, N.; Bachhav, N.; Rajanna, P. K.; Bhutani, H.; Paul, A. T.; Kumar, R. U.S. FDA Approved Drugs from 2015–June 2020: A Perspective. J. Med. Chem. 2021, 64, 2339–2381.
- 3
Marshall, C. M.; Federice, J. G.; Bell, C. N.; Cox, P. B.; Njardarson, J. T. An Update on the Nitrogen Heterocycle Compositions and Properties of U.S. FDA-Approved Pharmaceuticals (2013–2023). J. Med. Chem. 2024, 67, 11622–11655.
10.1021/acs.jmedchem.4c01122 Google Scholar
- 4 Jin, Z., Shen, G., Lv, X. Recent Advances in Domino Synthesis of Fused Polycyclic N-Heterocycles Based on Intramolecular Alkyne Hydroamination under Copper Catalysis. Chin. J. Chem. 2023, 41, 3751–3771.
- 5 Takada, N.; Iwatsuki, M.; Suenaga, K.; Uemura, D. Pinnamine, an alkaloidal marine toxin, isolated from Pinna muricata. Bioactive Alkaloids from the Sea: A Review. Tetrahedron Lett. 2000, 41, 6425–6428.
- 6 Kuramoto, M.; Arimoto, H.; Uemura, D. Bioactive Alkaloids from the Sea: A Review. Mar. Drugs 2004, 2, 39–54.
- 7 Wonnacott, S.; Gallagher, T. The Chemistry and Pharmacology of Anatoxin-a and Related Homotropanes with respect to Nicotinic Acetylcholine Receptors. Mar. Drugs 2006, 4, 228–254.
- 8 Dawood, R. S.; Chidipudi, S. R.; O'Connor, D. C.; Lewis, W.; Hamza, D.; Pearce, C.; Jones, A. G.; Wilkie, R. Georgiou, P. I.; Storr, T. E.; Moore, J. C.; Stockman, R. A. PdII-Mediated Oxidative Amination for Access to a 9-Azabicyclo[4.2.1]nonane Compound Library and Anatoxin-a. Eur. J. Org. Chem. 2018, 2018, 5558–5561.
- 9 Aronstam, R. S.; Witkop, B. Anatoxin-a Interactions with Cholinergic Synaptic Molecules. Proc. Natl. Acad. Sci. U. S. A. 1981, 78, 4639–4643.
- 10 Kanne, D. B.; Tomizawa, M.; Durkin, K. A.; Casida, J. E. 6′-Methylpyrido[3,4-b]norhomotropane: synthesis and outstanding potency in relation to the α4β2 nicotinic receptor pharmacophore model. Bioorg. Med. Chem. Lett. 2005, 15, 877–881.
- 11 de Sa Alves, F. R.; Barreiro, E. J.; Manssour Fraga, C. A. From Nature to Drug Discovery: The Indole Scaffold as a ‘Privileged Structure’. Mini-Rev. Med. Chem. 2009, 9, 782–793.
- 12 Robaa, D.; Enzensperger, C.; Abul Azm, S. E. D.; El Khawass, E. S.; El Sayed, O.; Lehmann, J. Dopamine Receptor Ligands. Part 18: Modification of the Structural Skeleton of Indolobenzazecine-Type Dopamine Receptor Antagonists. J. Med. Chem. 2010, 53, 2646–2650.
- 13 Liu, H.; Jia, Y. Ergot Alkaloids: Synthetic Approaches to Lysergic Acid and Clavine Alkaloids. Nat. Prod. Rep. 2017, 34, 411–432.
- 14 Scott, J. S.; Bailey, A.; Buttar, D.; Carbajo, R. J.; Curwen, J.; Davey, R. R. J.; Davies, R. D. M.; Degorce, S. L.; Donald, C.; Gangl, E.; Greenwood, R.; Groombridge, S. D.; Johnson, T.; Lamont, S.; Lawson, M.; Lister, A.; Morrow, C. J.; Moss, T. A.; Pink, J. H.; Polanski, R. Tricyclic Indazoles—A Novel Class of Selective Estrogen Receptor Degrader Antagonists. J. Med. Chem. 2019, 62, 1593–1608.
- 15 Lovering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756.
- 16 Galloway, W. R.; Isidro-Llobet, A.; Spring, D. R. Diversity-oriented Synthesis as a Tool for the Discovery of Novel Biologically Active Small Molecules. Nat. Commun. 2010, 1, 80.
- 17 Aldeghi, M.; Malhotra, S.; Selwood, D. L.; Chan, A. W. E. Two- and Three-dimensional Rings in Drugs. Chem. Biol. Drug Des. 2014, 83, 450–461.
- 18 Talele, T. T. Opportunities for Tapping into Three-Dimensional Chemical Space through a Quaternary Carbon. J. Med. Chem. 2020, 63, 13291–13315.
- 19
PhD, J. C. The Importance of Stereochemistry in Drug Action and Disposition. Clin. Pharmacol. 1992, 32, 925–929.
10.1002/j.1552-4604.1992.tb04640.x Google Scholar
- 20 Waldeck, B. Biological Significance of the Enantiomeric Purity of Drugs. Chirality 1993, 5, 350–355.
- 21 Rentsch, K. M. The Importance of Stereoselective Determination of Drugs in the Clinical Laboratory. J. Biochem. Biophys. Methods 2002, 54, 1–9.
- 22 Kim, U. B.; Jung, D. J.; Jeon, H. J.; Rathwell, K.; Lee, S.-g. Synergistic Dual Transition Metal Catalysis. Chem. Rev. 2020, 120, 13382–13433.
- 23 Allen, A. E.; MacMillan, D. W. C. Synergistic Catalysis: A Powerful Synthetic Strategy for New Reaction Development. Chem. Sci. 2012, 3, 633–658.
- 24 Lin, L.; Feng, X. Catalytic Strategies for Diastereodivergent Synthesis. Chem. - Eur. J. 2017, 23, 6464–6482.
- 25 Huo, X.; Li, G.; Wang, X.; Zhang, W. Bimetallic Catalysis in Stereodivergent Synthesis. Angew. Chem. Int. Ed. 2022, 61, e202210086.
- 26 Wei, L.; Wang, C.-J. Asymmetric Transformations Enabled by Synergistic Dual Transition-metal Catalysis. Chem. Catal. 2023, 3, 100455.
- 27 Chen, D.-F.; Gong, L.-Z. Organo/Transition-Metal Combined Catalysis Rejuvenates Both in Asymmetric Synthesis. J. Am. Chem. Soc. 2022, 144, 2415–2437.
- 28 Wei, L.; Fu, C.; Wang, Z.-F.; Tao, H.-Y.; Wang, C.-J. Synergistic Dual Catalysis in Stereodivergent Synthesis. ACS Catal. 2024, 14, 3812–3844.
- 29 Sawamura, M.; Sudoh, M.; Ito, Y. An Enantioselective Two-Component Catalyst System: Rh-Pd-Catalyzed Allylic Alkylation of Activated Nitriles. J. Am. Chem. Soc. 1996, 118, 3309–3310.
- 30 Sammis, G. M.; Danjo, H.; Jacobsen, E. N. Cooperative Dual Catalysis: Application to the Highly Enantioselective Conjugate Cyanation of Unsaturated Imides. J. Am. Chem. Soc. 2004, 126, 9928–9929.
- 31 Krautwald, S.; Sarlah, D.; Schafroth, M. A.; Carreira, E. M. Enantio- and Diastereodivergent Dual Catalysis: α-Allylation of Branched Aldehydes. Science 2013, 340, 1065–1068.
- 32 Huo, X.; He, R.; Zhang, X.; Zhang, W. An Ir/Zn Dual Catalysis for Enantio- and Diastereodivergent α-Allylation of α-Hydroxyketones. J. Am. Chem. Soc. 2016, 138, 11093–11096.
- 33 Cruz, F. A.; Dong, V. M. Stereodivergent Coupling of Aldehydes and Alkynes via Synergistic Catalysis Using Rh and Jacobsen's Amine. J. Am. Chem. Soc. 2017, 139, 1029–1032.
- 34 Jiang, X.; Beiger, J. J.; Hartwig, J. F. Stereodivergent Allylic Substitutions with Aryl Acetic Acid Esters by Synergistic Iridium and Lewis Base Catalysis. J. Am. Chem. Soc. 2017, 139, 87–90.
- 35 Huo, X.; He, R.; Fu, J.; Zhang, J.; Yang, G.; Zhang, W. Stereoselective and Site-Specific Allylic Alkylation of Amino Acids and Small Peptides via a Pd/Cu Dual Catalysis. J. Am. Chem. Soc. 2017, 139, 9819–9822.
- 36 Wei, L.; Xu, S.-M.; Zhu, Q.; Che, C.; Wang, C.-J. Synergistic Cu/Pd Catalysis for Enantioselective Allylic Alkylation of Aldimine Esters: Access to α,α-Disubstituted α-Amino Acids. Angew. Chem. Int. Ed. 2017, 56, 12312–12316.
- 37 Huo, X.; Zhang, J.; Fu, J.; He, R.; Zhang, W. Ir/Cu Dual Catalysis: Enantio- and Diastereodivergent Access to α,α-Disubstituted α-Amino Acids Bearing Vicinal Stereocenters. J. Am. Chem. Soc. 2018, 140, 2080–2084.
- 38 Wei, L.; Zhu, Q.; Xu, S.-M.; Chang, X.; Wang, C.-J. Stereodivergent Synthesis of α,α-Disubstituted α-Amino Acids via Synergistic Cu/Ir Catalysis. J. Am. Chem. Soc. 2018, 140, 1508–1513.
- 39 Zhang, Q.; Yu, H.; Shen, L.; Tang, T.; Dong, D.; Chai, W.; Zi, W. Stereodivergent Coupling of 1,3-Dienes with Aldimine Esters Enabled by Synergistic Pd and Cu Catalysis. J. Am. Chem. Soc. 2019, 141, 14554–14559.
- 40 He, R.; Huo, X.; Zhao, L.; Wang, F.; Jiang, L.; Liao, J.; Zhang, W. Stereodivergent Pd/Cu Catalysis for the Dynamic Kinetic Asymmetric Transformation of Racemic Unsymmetrical 1,3-Disubstituted Allyl Acetates. J. Am. Chem. Soc. 2020, 142, 8097–8103.
- 41 Peng, L.; He, Z.; Xu, X.; Guo, C. Cooperative Ni/Cu-Catalyzed Asymmetric Propargylic Alkylation of Aldimine Esters. Angew. Chem. Int. Ed. 2020, 59, 14270–14274.
- 42 Qi, J.; Wei, F.; Tung, C. H.; Xu, Z. Modular Synthesis of α-Quaternary Chiral β-Lactams by a Synergistic Copper/Palladium-Catalyzed Multicomponent Reaction. Angew. Chem. Int. Ed. 2021, 60, 13814–13818.
- 43 Xiao, L.; Wei, L.; Wang, C.-J. Stereodivergent Synthesis of Enantioenriched γ-Butyrolactones Bearing Two Vicinal Stereocenters Enabled by Synergistic Copper and Iridium Catalysis. Angew. Chem. Int. Ed. 2021, 60, 24930–24940.
- 44 Yang, S. Q.; Wang, Y. F.; Zhao, W. C.; Lin, G. Q.; He, Z. T. Stereodivergent Synthesis of Tertiary Fluoride-Tethered Allenes via Copper and Palladium Dual Catalysis. J. Am. Chem. Soc. 2021, 143, 7285–7291.
- 45 He, Z.; Peng, L.; Guo, C. Catalytic Stereodivergent Total Synthesis of Amathaspiramide D. Nat. Synth. 2022, 1, 393–400.
- 46 Xu, Y.; Wang, H.; Yang, Z.; Zhou, Y.; Liu, Y.; Feng, X. Stereodivergent Total Synthesis of Rocaglaol Initiated by Synergistic Dual-metal-catalyzed Asymmetric Allylation of Benzofuran-3(2H)-one. Chem 2022, 8, 2011–2022.
- 47 Zhang, J.; Huo, X.; Xiao, J.; Zhao, L.; Ma, S.; Zhang, W. Enantio- and Diastereodivergent Construction of 1,3-Nonadjacent Stereocenters Bearing Axial and Central Chirality through Synergistic Pd/Cu Catalysis. J. Am. Chem. Soc. 2021, 143, 12622–12632.
- 48 Zhang, J.; Luo, Y.; Zheng, E.; Huo, X.; Ma, S.; Zhang, W. Synergistic Pd/Cu-Catalyzed 1,5-Double Chiral Inductions. J. Am. Chem. Soc. 2024, 146, 9241–9251.
- 49 Dong, W. W.; Tian, K.; Dong, X. Q.; Wang, C. J., Design, Synthesis and Application of Multifunctional Chiral Aminophosphine Catalyst for Asymmetric Intermolecular Cross Rauhut-Currier Reaction. Chin. J. Chem. 2022, 40, 2061–2066.
- 50 Xiao, L.; Li, B.; Xiao, F.; Fu, C.; Wei, L.; Dang, Y.; Dong, X.-Q.; Wang, C.-J. Stereodivergent Synthesis of Enantioenriched Azepino[3,4,5-cd]- indoles via Cooperative Cu/Ir-catalyzed Asymmetric Allylic Alkylation and Intramolecular Friedel-Crafts Reaction. Chem. Sci. 2022, 13, 4801–4812.
- 51 Molander, G. A. Diverse Methods for Medium Ring Synthesis. Acc. Chem. Res. 1998, 31, 603–609.
- 52 Galli, C.; Mandolini, L. The Role of Ring Strain on the Ease of Ring Closure of Bifunctional Chain Molecules. Eur. J. Org. Chem. 2000, 2000, 3117–3125.
- 53Deposition numbers 2395557–2395561 (for compounds (6S,7S,10S)- 6a, (6R,7R,10R)-6a, (6S,7R,10R)-6a, (6R,7S,10S)-6a, and (S,S)-8) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre.
- 54 Hashimoto, M.; Terashima, S. A novel synthesis of the C1-C17 fragment of carzinophilin. Tetrahedron Lett. 1994, 35, 9409–9412.
- 55 Chang, X.; Yang, Y.; Shen, C.; Xue, K.-S.; Wang, Z.-F.; Cong, H.; Tao, H.-Y.; Chung, L. W.; Wang, C.-J. β-Substituted Alkenyl Heteroarenes as Dipolarophiles in the Cu(I)-Catalyzed Asymmetric 1,3-Dipolar Cycloaddition of Azomethine Ylides Empowered by a Dual Activation Strategy: Stereoselectivity and Mechanistic Insight. J. Am. Chem. Soc. 2021, 143, 3519–3535.