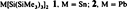Journal list menu
Export Citations
Download PDFs
Cover Picture (Angew. Chem. Int. Ed. Engl. 12/1995)
- First Published: July 7, 1995
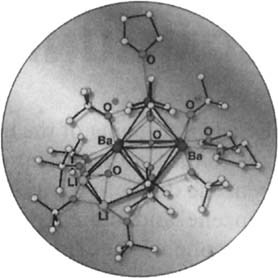
The cover picture shows the crystal structure of the elevenfold positively charged cation [Ba6Li3O2]11+, in which a Ba6O octahedron and a Li3Ba3O prismane are connected through a shared Ba3 triangular face. A density functional calculation of 33 centers and 460 electrons (!) suggests that this is a thermodynamically favorable polyion aggregate containing weakly coupled O2- centers. Eleven tert-butyl alcoholate anions and three tetrahydrofuran solvent molecules provide kinetic shielding. The lipophilic wrapping by the largely impenetrable hydrocarbon skin (C56H123) anchored by O centers also limits the cluster size, as is confirmed by the structure of the hexameric sodium tetraphenylimidodiphosphate, which in its outer sphere contains 24 phenyl rings. The lipophilically wrapped polyion aggregates form by a self-organization principle for sterically overcrowded molecules so far not systematically exploited, in which the size of the thermodynamically stabilizing polyion core depends on the space within its lipophilic covering. A more detailed report is provided by H. Bock, E. Herrmann, N. Rösch, et al. on pages 1353 and 1355. The cover picture was prepared by R. Utermark, Hoechst AG (Frankfurt am Main, Germany).
Graphical Abstract (Angew. Chem. Int. Ed. Engl. 12/1995)
- Pages: 1261-1266
- First Published: July 7, 1995
Reviews
Vibrational Spectroscopy in Supercritical Fluids: From Analysis and Hydrogen Bonding to Polymers and Synthesis†
- Pages: 1275-1295
- First Published: July 7, 1995
Not magic solvents but materials with a wide range of applications, supercritical fluids have already made a major impact on many areas of chemistry. The problems associated with reactions in supercritical fluids are becoming increasingly more manageable. Thus for example, Soxhlet extraction in the analytical laboratory is rapidly being replaced by extraction with supercritical fluids. Vibrational spectroscopy, particularly IR spectroscopy with its higher sensitivity, is playing a crucial role in probing chemistry in supercritical fluids; processes can be monitored, reaction conditions optimized, and products analyzed.
Recent Trends in Photoaffinity Labeling
- Pages: 1296-1312
- First Published: July 7, 1995
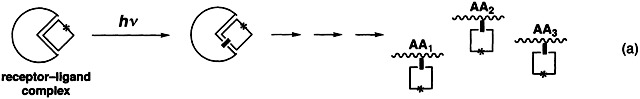
Site-directed and efficient labeling of amino acids in biological receptors is possible with ligand analogues that form reactive intermediates on irradiation. This approach was used to label the binding sites of many receptors, for example by photoinduced coupling with (radioactive) nitrenes, carbocations, carbenes, and excited ketones [Eq. (a)]. The labeled amino acids are identified by proteolysis of the complex, purification, and sequencing. This method is well-suited for the study of ligand—receptor interactions and provides useful information for site-directed mutagenesis. □ = ligand, * = radioactive label, AA1, AA2, AA3 = amino acids.
Highlights
Sulfuric Acid from Sulfur Trioxide and Water—A Surprisingly Complex Reaction†
- Pages: 1313-1315
- First Published: July 7, 1995
On paper very simple, but in reality far more complicated—this applies to the formation of sulfuric acid in the atmosphere, which leads to the environmental problem of acid rain. In this reaction sulfur trioxide is transformed into sulfur dioxide, which reacts with water dimers to give sulfuric acid. Ab initio calculations as well as spectroscopic and kinetic studies have yielded new information on the reaction mechanism.
Palladium-Catalyzed Amination of Aryl Halides—Catalysts on New Routes to Known Targets
- Pages: 1316-1317
- First Published: July 7, 1995
It's the base that matters! Sodium tert-butoxide can be used to facilitate Pd-catalyzed CN couplings directly with the amine. This was shown by research by Hartwig et al. and Buchwald et al. Thus, the use of ecologically unsound tin amides in the hetero-Heck reaction may be avoided in future. Further improvement of the catalysts to make them industrially more interesting, and the extension of the method to CO couplings is eagerly awaited.
Communications
First-Ever Per(onio) Substitution of Benzene: The Role of the Counterion
- Pages: 1319-1321
- First Published: July 7, 1995
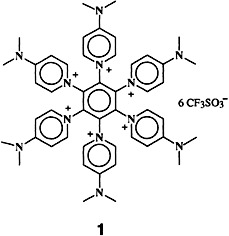
Efficient trapping of the liberated fluoride ions is the key to the synthesis of the hexakis(onio)-substituted benzene derivative 1 in 92% yield from hexafluorobenzene and dimethylaminopyridine. In the solid state one of the triflate counterions of 1 is located above and one below the central benzene ring to give a triple-decker-type structure.
η5:η2 Coordination of a cyclo-E5 Ligand, EP, As†‡
- Pages: 1321-1323
- First Published: July 7, 1995

An unusual, additional side-on coordination of an iridium center and a rhodium complex fragment with 16 valence electrons is realized in the new compounds 1. Compounds 1a, E = P, M = Ir, and 1b, E = As, M = Rh, were prepared from the sandwich complexes [Cp*Fe(η5-E5)]. The isomers of 1a and 1b with terminal η1 coordination of the metal complex fragment have been obtained as well.
The First Clusters with a Y-Shaped Arrangement of Ligands at Three-Coordinate RhI Atoms in the Solid State: [M1M2{μ-P(C6H11)2}(CO)8Rh(PPh3)] (M1, M2 = Mn, Re)
- Pages: 1325-1327
- First Published: July 7, 1995
![The First Clusters with a Y-Shaped Arrangement of Ligands at Three-Coordinate RhI Atoms in the Solid State: [M1M2{μ-P(C6H11)2}(CO)8Rh(PPh3)] (M1, M2 = Mn, Re)](/cms/asset/f8aa5d33-cb05-450c-8562-09e0547af608/must001.jpg)
Formally only 12 valence electrons are assigned to the RhI atom in the cyclo-M3 clusters in the title. This electron deficiency is compensated by π-electron delocalization in the M1-Rh-M2 three-membered ring, and the unusual threefold coordination of the d8-RhI atom (see picture on the right) is thus stabilized. The cluster can be synthesized in good yields from [Rh(CO)(PPh3)]Cl and PPh4[M1M2{μ-P(C6H11)2} CO8].
Thymidine Diphospho-6-deoxy-α-D-ribo-3-hexulose—Synthesis of a Central Intermediate in the Biosynthesis of Di- and Trideoxysugars†
- Pages: 1328-1329
- First Published: July 7, 1995
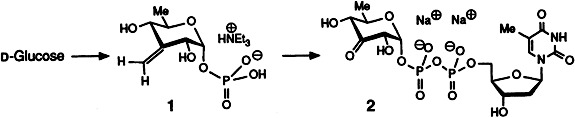
An indirect strategy was needed for the first synthesis of a labile dNDP-3-keto sugar, the title compound 2. D-Glucose is converted into methylene phosphate 1, which after ozonolysis and linkage of thymidine monophosphate, provides 2. According to present knowledge the target compound is an important intermediate in the biosynthesis of dideoxy, trideoxy, aminodeoxy, and branched sugars.
S7NH and S7NCH3 as Novel Chelating Ligands in [(η5-C5H5)2Ti] Complexes: Synthesis of Medium-Sized Sulfur–Nitrogen Rings by Ligand Transfer†
- Pages: 1330-1331
- First Published: July 7, 1995
A Camouflaged Icosahedral Carborane: Dodecamethyl-1,12-dicarba-closo-dodecaborane(12) and Related Compounds†
- Pages: 1332-1334
- First Published: July 7, 1995

Complete B-methylation is possible for 1,12-dicarba-closo-dodecaborane(12) derivatives that are substituted on one or both carbon atoms. Electrophilic substitution under harsh conditions affords rigid “hydrocarbon balls” in high yields. An example of these carboranes, the title compound (shown as a space-filling model on the right), has twelve methyl groups arranged in an icosahedral manner and supported on a carborane skeleton.
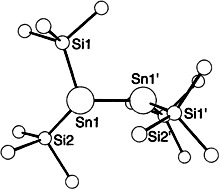
Bis(hypersilyl)tin and Bis(hypersilyl)lead, Two Electron-Rich Carbene Homologs†‡
- Pages: 1334-1336
- First Published: July 7, 1995
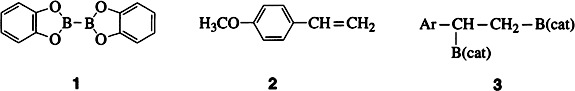
Transition Metal Catalyzed Diboration of Vinylarenes†‡
- Pages: 1336-1338
- First Published: July 7, 1995
1,5-Anhydrohexitol Nucleic Acids, a New Promising Antisense Construct†
- Pages: 1338-1339
- First Published: July 7, 1995
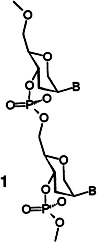
Both natural oligoribonucleotides and oligodeoxynucleotides are hybridized sequence-selectively by hexitol nucleic acids (HNA) 1, which are composed of 1,5-anhydrohexitol nucleoside analogues linked by phosphate groups. Owing to the strongly increased duplex stability, these constructs should be pursued for potential applications as antisense agents.
Diborylcarbenes as Reactive Intermediates in Double 1,2-Rearrangements with Low Activation Enthalpies†‡
- Pages: 1340-1343
- First Published: July 7, 1995
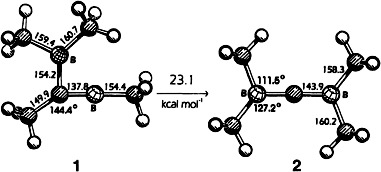
Despite three neighboring electron-deficient centers, dicarborylcarbenes are not too high in energy to be accessible thermally. This was shown by the low activation enthalpies (≈22–23 kcal mol−1) of double 1,2-rearrangements of borylmethyleneboranes, in which alkyl groups migrate from carbon to boron and aryl groups from boron to carbon. Ab initio calculations reveal that tetramethyldiborylcarbene 2 is only 23.1 kcal mol−1 higher in energy than tetramethylborylmethyleneborane 1.
Ring Expansion of the Fullerene Core by Highly Regioselective Formation of Diazafulleroids†
- Pages: 1343-1345
- First Published: July 7, 1995
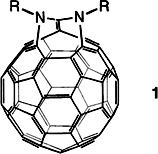
Fullerenes with enlarged openings such as diazafulleroid 1 are obtained by twofold addition of azides to C60. Even after cleavage of the two 5–6 bonds and introduction of the imino bridges, the π-electron system of C60 remains intact. Compound 1 contains three seven-membered rings and one eleven-membered ring, and may hold the key to the synthesis of endohedral fullerenes under mild conditions.
Synthesis of a Dynemicin A Analogue and Its Bergaman-Type Cycloaromatization†
- Pages: 1345-1348
- First Published: July 7, 1995
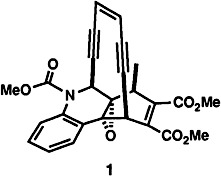
An efficient and simple approach is described here for the synthesis of enediyne 1, a model compound for the antibacterial and anticancer agent dynamicin A. Compound 1 undergoes the cycloaromatization reaction characteristic of such enediyne systems and can conceivably be attached to various “drug delivery systems” through the two ester groups.
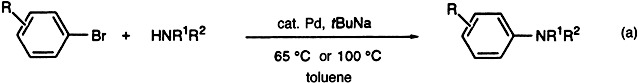
A Simple Catalytic Method for the Conversion of Aryl Bromides to Arylamines†
- Pages: 1348-1350
- First Published: July 7, 1995
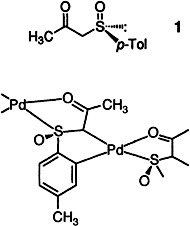
Enantiomerically Pure Palladacycles Derived from β-Ketosulfoxides†
- Pages: 1351-1353
- First Published: July 7, 1995
The Lipophilically Wrapped Polyion Aggregate {[Ba6Li3O2]11+ [−OC(CH3)3]11(OC4H8)3}, a Face-Sharing (Octahedron + Prismane) Ba6Li3O2 Polyhedron in a Hydrocarbon Ellipsoid: Preparation, Single Crystal Structure Analysis, and Density Functional Calculations†‡
- Pages: 1353-1355
- First Published: July 7, 1995
![The Lipophilically Wrapped Polyion Aggregate {[Ba6Li3O2]11+ [−OC(CH3)3]11(OC4H8)3}, a Face-Sharing (Octahedron + Prismane) Ba6Li3O2 Polyhedron in a Hydrocarbon Ellipsoid: Preparation, Single Crystal Structure Analysis, and Density Functional Calculations](/cms/asset/4a6c6949-0b9f-4efe-8338-fcb8e28e6750/must001.jpg)
An amazingly simple access by dissolving barium metal in tBuOH, addition of nBuLi, and crystallization from THF/hexane leads to the polyion aggregate 1. The novel polyhedral Ba6Li3O2 core has a charge of 11+ and is surrounded by an ellipsoidal C56H123 hydrocarbon skin. Density functional calculations for a 33-center/460-electron model revealed that it is indeed a polyion aggregate, and that weak interactions exist between the two oxygen centers in the polyhedron. In the second communication the hydrocarbon ellipsoid of the polyion aggregate consists of 24 phenyl groups, and the interactions between them are analyzed.
The Lipophilically Wrapped Polyion Aggregate {H120C144O24(OP)2N−Na }, a Hexameric Sodium Tetraphenyl Imidodiphosphate Containing an [Na6O12] Core in a Hydrocarbon Ellipsoid†‡
}, a Hexameric Sodium Tetraphenyl Imidodiphosphate Containing an [Na6O12] Core in a Hydrocarbon Ellipsoid†‡
- Pages: 1355-1357
- First Published: July 7, 1995
![The Lipophilically Wrapped Polyion Aggregate {H120C144O24(OP)2N−Na}, a Hexameric Sodium Tetraphenyl Imidodiphosphate Containing an [Na6O12] Core in a Hydrocarbon Ellipsoid](/cms/asset/c3904184-94f4-4143-9b0a-1a8d9c7b96b2/must001.jpg)
An amazingly simple access by dissolving barium metal in tBuOH, addition of nBuLi, and crystallization from THF/hexane leads to the polyion aggregate 1. The novel polyhedral Ba6Li3O2 core has a charge of 11+ and is surrounded by an ellipsoidal C56H123 hydrocarbon skin. Density functional calculations for a 33-center/460-electron model revealed that it is indeed a polyion aggregate, and that weak interactions exist between the two oxygen centers in the polyhedron. In the second communication the hydrocarbon ellipsoid of the polyion aggregate consists of 24 phenyl groups, and the interactions between them are analyzed.
Aromatic Gallium Heterocycles: Synthesis of the First Gallatabenzene†‡
- Pages: 1357-1359
- First Published: July 7, 1995
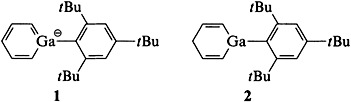
Deprotonation of the conjugate acid 2 led to the first gallatabenzene 1. The acidity of 2 and the low-field shift of the NMR signals of the ring protons of 1 relative to the corresponding properties of cyclohexadienide suggest that 1 has aromatic character. The heterocycle of 1 can function as a ligand and was coordinated to a Mn(CO)3 fragment.
Metal Oxo Cation Receptors: Multimode Coordination to the Dioxoosmium(VI) Cation†
- Pages: 1359-1362
- First Published: July 7, 1995
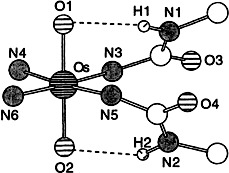
New tetraamidato ligands with appended urea moieties have the requisite electrochemical and structural properties to stabilize the dioxoosmium(IV) cation through multiple interactions. In the corresponding 1:1 complexes four strong bonds between metal and nitrogen donor and an additional hydrogen bond between each oxo ligand and a C(O)NHR moiety are responsible for the stability. The primary coordination sphere of an osmium complex of this type is shown on the right.
S(NPMe3)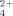 : The First Structure Determination of a Dication of Hexavalent Sulfur†
: The First Structure Determination of a Dication of Hexavalent Sulfur†
- Pages: 1362-1363
- First Published: July 7, 1995
Attraction of the charges on the participant atoms causes the polar covalent NS and NP single bonds in the cation of the phosphorane iminato complex [S(NPMe3)4]Cl2 (shown on the right) to approach the lengths of double bonds. This is in agreement with the results of ab initio calculations. The complex was obtained from S2Cl2 and the silylated phosphorane imine Me3SiNPMe3 in acetonitrile.
“Cation-π Interactions” Detected by Mass Spectrometry; Selective Recognition of Alkali Metal Cations by a π-Basic Molecular Cavity
- Pages: 1364-1366
- First Published: July 7, 1995
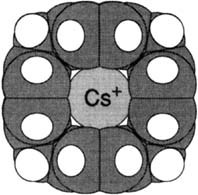
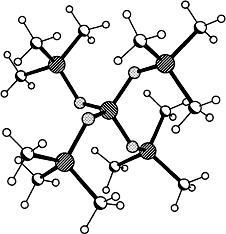
A large, soft Cs+ ion nests in the cavity of [1.1.1]-paracyclophane (1), giving rise to a stable [1 · Cs]+ complex (space-filling model on the right), which can be detected in the gas phase by mass spectrometry. Crucial to the formation of this complex is the interaction of the Cs+ ion with all four arene rings. The somewhat smaller Rb+ ion also forms a stable complex with 1, but Li+, Na+, and K+ are too small to interact effectively with the cavity of 1.
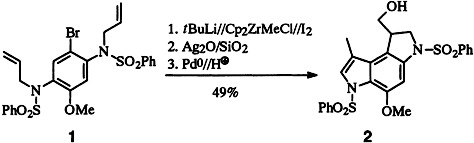
Efficient Total Synthesis of the Pharmacophore of the Anticancer Antibiotic CC-1065 by Zirconocene- and Palladium-Initiated Cyclizations†‡
- Pages: 1366-1368
- First Published: July 7, 1995
Corrigenda
Book Reviews
Book Review: Protection Rackets?: Tactics of Organic Synthesis. By Tse-Lok Ho
- Pages: 1369-1370
- First Published: July 7, 1995
Book Review: Protecting Groups. By P. J. Kocienski
- Page: 1370
- First Published: July 7, 1995
Book Review: Der große Schwindel. By F. DiTrocchio
- Pages: 1370-1372
- First Published: July 7, 1995
Book Review: Stereoselective Synthesis. 2nd Edition. By M. Nógrádi
- Page: 1372
- First Published: July 7, 1995




![S7NH and S7NCH3 as Novel Chelating Ligands in [(η5-C5H5)2Ti] Complexes: Synthesis of Medium-Sized Sulfur–Nitrogen Rings by Ligand Transfer](/cms/asset/dfaa3d0f-73fe-408a-a8c9-9be808b10e7b/must001.jpg)