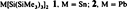Bis(hypersilyl)tin and Bis(hypersilyl)lead, Two Electron-Rich Carbene Homologs†‡
Corresponding Author
Dr. Karl Wihelm Klinkhammer
Institut für Anorganische Chemie der Universität, Pfaffenwaldring 55, D-70550 Stuttgart (Germany), Telefax: Int. code + (711) 685-4241, e-mail: [email protected]
Institut für Anorganische Chemie der Universität, Pfaffenwaldring 55, D-70550 Stuttgart (Germany), Telefax: Int. code + (711) 685-4241, e-mail: [email protected]Search for more papers by this authorDr. Wolfgang Schwarz
Institut für Anorganische Chemie der Universität, Pfaffenwaldring 55, D-70550 Stuttgart (Germany), Telefax: Int. code + (711) 685-4241, e-mail: [email protected]
Search for more papers by this authorCorresponding Author
Dr. Karl Wihelm Klinkhammer
Institut für Anorganische Chemie der Universität, Pfaffenwaldring 55, D-70550 Stuttgart (Germany), Telefax: Int. code + (711) 685-4241, e-mail: [email protected]
Institut für Anorganische Chemie der Universität, Pfaffenwaldring 55, D-70550 Stuttgart (Germany), Telefax: Int. code + (711) 685-4241, e-mail: [email protected]Search for more papers by this authorDr. Wolfgang Schwarz
Institut für Anorganische Chemie der Universität, Pfaffenwaldring 55, D-70550 Stuttgart (Germany), Telefax: Int. code + (711) 685-4241, e-mail: [email protected]
Search for more papers by this authorWe thank Prof. Dr. G. Becker for his splendid support.– For the term “hypersilyl” see citation 5 in ref. [10].
Dedicated to Professor Peter I. Paetzold on the occasion of his 60th birthday
Graphical Abstract
References
- 1 T. Fjeldberg, A. Haaland, B. E. R. Schilling, M. F. Lappert, A. J. Thorne, J. Chem. Soc. Dalton. Trans. 1986, 1551.
- 2 C. D. Schafeffer, Jr., J. J. Zuckerman, J. Am. Chem. Soc. 1974, 96, 7160; D. E. Goldberg, D. H. Harris, M. F. Lappert, K. M. Thomas, J. Chem. Soc. Chem. Commun. 1976, 261; M. F. Lappert, P. P. Power, M. J. Slade, L. Hedberg, V. Schomaker, J. Chem. Soc. Chem. Commun. 1979 369.
- 3 H. Grützmacher, H. Pritzkow, F. T. Edelmann, Organometallics 1991, 10, 23; S. Brooker, J.-K. Buijink, F. T. Edelmann, Organometallics 1991, 10, 25.
- 4 M. Weidenbruch, J. Schlaefke, A. Schäfer, K. Peters, H. G. von Schnering, H. Marsmann, Angew. Chem. 1994, 106, 1938; Angew. Chem. Int. Ed. Engl. 1994, 33, 1846.
- 5(a) W.-W. du Mont, H.-J. Kroth, Angew. Chem. 1977, 89, 832; Angew. Chem. Int. Ed. Engl. 1977, 16, 792; (b) S. C. Goel, M. Y. Chiang, D. J. Rauscher, W. E. Buhro, J. Am. Chem. Soc. 1993, 115, 160; (c) M. Westerhausen, M. M. Enzelberger, W. Schwarz, J. Organomet. Chem. 1995, 485, 185.
- 6Review: M. F. Lappert, Main Group Met. Chem. 1994, 17, 183.
- 7For ab initio calculations on the stability of dimers see for example: (a) G. J. Trinquier, J. Am. Chem. Soc. 1990, 112, 1039; (b) T. L. Windus, M. S. Gordon, J. Am. Chem. Soc. 1992, 114, 9559.
- 8 S. P. Mallela, I. Bernal, R. A. Geanangel, Inorg. Chem. 1992, 31, 1626.
- 9 S. P. Mallela and R. A. Geanangel claimed the isolation of a colorless(!) THF adduct of 1[ Inorg. Chem. 1990, 29, 3525]. Our own results (1 forms a brown THF solution) and the reported exterme 119Sn highfield shift led us to doubt its identity. (b) A. A. Arif, A. H. Cowley, T. M. Elkins, J. Organomet. Chem. 1987, 325, C11.
- 10 S. Henkel, K. W. Klinkhammer, W. Schwarz, Angew. Chem. 1994, 106, 721; Angew. Chem. Int. Ed. Engl. 1994, 33, 681. For earlier usage of bis(trimethylsilyl)amides in the syntheses of stannylenes and plumbylenes see for example ref. [6].
- 11 K. W. Klinkhammer, W. Schwarz, Z. Anorg. Allg. Chem. 1993, 619, 1777; K. W. Klinkhammer, W. Schwarz, J. Am. Chem. Soc., submitted.
- 12 The reaction of bis[bis(trimethylsily)amides] with hypersilanides is strongly dependent on the temperature and the solvent. Larger quantities of unknown paramagnetic compounds are formed in pentane above −30 °C. In the presence of toluene additional interesting products are found: At temperatures below -30 °C mainly solvated alkali metal tris[tris(trimethylsilyl)silyl]stannanides or -plumbanides are obtained in addition to 1 and 2, respectively. At higher temperatures the main products are alkali metal derivatives of benzylbis[tris(trimethylsilyl)silyl]stannane or -plumbane.
- 13(a) How much distannene (1)2 is already present in the triplet state at room temperature remains an unclarified question. In the EPR spectrum of the solid, no triplet resonances were observed in the temperature range between 4 and 300 K. However, ab initio calculations performed on the model system (H3Si)2Sn–Sn(SiH3)2 reveal only a small energy difference of about 25 kJ mol−1 in favor of the singlet state [13b] in the observed Si2Sn–SnSi2 backbone conformation in the solid, so that a measurable population of the triplet state at room temperature cannot be excluded. (b) MP2; quasi-relativistic pseudopotentials on Sn(46e core) and Si(10e core) and appropriate basis sets from G. Igel-Mann, H. Stoll, H. Preuss, Mol. Phys. 1988, 65, 1321; basis sets for H from T. H. Dunning, J. Chem. Phys. 1970, 19, 553.
- 14
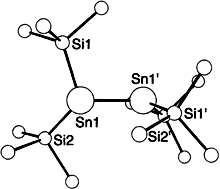
Crystal structure analyses: (1)2: a = 27.884(3) b = 13.106(1), c = 23.173(2) Å, β = 126.203(6)°, V = 6833.5(1) Å3, Z = 4, monoclinic, space group C2/c (no. 15), F(000) = 2592, ρcalcd = 1.194 g cm−3, μ = 1.03 mm−1, four-circle diffractometer P21 (Syntex), 3° <2θ<56°, MoKα, Wyckoff scan, T = –100 °C, N(hkl) = 8519, from which 7364> –1σ(I), Lorenz- and polarization correction, no absorption correction. The structure was solved by direct methods (SHELXS-86) refined with full-matrix least squares based on F
 values (SHELXL93) ((1)2 surprisngly crystallizes isotypically to tetrakis(hypersilyl)dithallium(Tl–Tl) which comprises two valence electrons less [10]). Sn, Si, and C atoms were refined anisotropically; the hydrogen atoms, except those which belong to a disordered trimethylsiyl group were found und their positional parameters were freely refined. The tin atom is slightly disordered; the occupation factor for the major positon refined to 0.9812(9). 433 parameters, 57 restraints, residual electron density: 0.776/-0.621 e Å−3, R1[F0 >4σ(F0)] = 0.044, wR2 = 0.091, GOF = 1.10. 2: a = 16.222(3), b = 22.130(3), c = 22.380(4) Å, α = 110.61(3), β = 100.57(3), γ = 100.35(3)°, V = 7127(1) Å3, Z = 8, triclinic, space group P−1 (no. 2), F(000) = 2848, ρcalcd = 1.309 g cm−3. μ = 5.04 mm−1, four-circle diffractometer P21 (Syntex), 6.5°<2θ<45°, MoKα, Wyckoff scan, T = −100 °C, N(hkl) = 18628, from which 18406>−3σ(I), Lorenz and polarization correction, empirical absorption correction (ϕ scan, min./max. transmission: 0.63/0.99). All the examined crystals were twinned about (1 0 1¯) by pseudomeroedry. In the individual used for the final structure solution, the refined volume proportion of the minority component was however very small [0.131(5)]. The structure was solved by direct methods (SHELXS-86) refined with full-matrix least squares based on F
values (SHELXL93) ((1)2 surprisngly crystallizes isotypically to tetrakis(hypersilyl)dithallium(Tl–Tl) which comprises two valence electrons less [10]). Sn, Si, and C atoms were refined anisotropically; the hydrogen atoms, except those which belong to a disordered trimethylsiyl group were found und their positional parameters were freely refined. The tin atom is slightly disordered; the occupation factor for the major positon refined to 0.9812(9). 433 parameters, 57 restraints, residual electron density: 0.776/-0.621 e Å−3, R1[F0 >4σ(F0)] = 0.044, wR2 = 0.091, GOF = 1.10. 2: a = 16.222(3), b = 22.130(3), c = 22.380(4) Å, α = 110.61(3), β = 100.57(3), γ = 100.35(3)°, V = 7127(1) Å3, Z = 8, triclinic, space group P−1 (no. 2), F(000) = 2848, ρcalcd = 1.309 g cm−3. μ = 5.04 mm−1, four-circle diffractometer P21 (Syntex), 6.5°<2θ<45°, MoKα, Wyckoff scan, T = −100 °C, N(hkl) = 18628, from which 18406>−3σ(I), Lorenz and polarization correction, empirical absorption correction (ϕ scan, min./max. transmission: 0.63/0.99). All the examined crystals were twinned about (1 0 1¯) by pseudomeroedry. In the individual used for the final structure solution, the refined volume proportion of the minority component was however very small [0.131(5)]. The structure was solved by direct methods (SHELXS-86) refined with full-matrix least squares based on F values (SHELXL93). Pb. Si, and C atoms were refined anisotorpically: the hydrogen atoms were calculated at ideal positions and included in the refinement according to riding model (AFIX 137) with fixed isotopic displacement parameters. In two out of four symmetry independent molecules the lead atom shows a positional disorder which was carried by a split model calculation. 1068 parameters. 64 restraints, residual electron density: 1.874/-1.074 e Å−3, R1[F0 >4σ(F0)] = 0.067, wR2 = 0.141, GOF = 1.10. Further details of the crystal structure investigations may be obtained from the Fachinformationszentrum Karlsruhe, D-76344 Eggenstein-Leopoldshafen (Germany), on quoting the depository numbers CSD-141531 (1)2 and CSD-141530 (2).
values (SHELXL93). Pb. Si, and C atoms were refined anisotorpically: the hydrogen atoms were calculated at ideal positions and included in the refinement according to riding model (AFIX 137) with fixed isotopic displacement parameters. In two out of four symmetry independent molecules the lead atom shows a positional disorder which was carried by a split model calculation. 1068 parameters. 64 restraints, residual electron density: 1.874/-1.074 e Å−3, R1[F0 >4σ(F0)] = 0.067, wR2 = 0.141, GOF = 1.10. Further details of the crystal structure investigations may be obtained from the Fachinformationszentrum Karlsruhe, D-76344 Eggenstein-Leopoldshafen (Germany), on quoting the depository numbers CSD-141531 (1)2 and CSD-141530 (2).
- 15 S. P. Mallela, R. A. Geanangel, Inorg. Chem. 1993, 32, 602.
- 16For examples see: M. Kira, T. Maruyama, C. Kabuto, K. Ebata, H. Sakurai, Angew. Chem. 1994, 106, 1575; Angew. Chem. Int. Ed. Engl. 1994, 33, 1489, and references therein.
- 17See for example: S. P. Mallela, R. A. Geannangel, Inorg. Chem. 1993, 32, 5623.