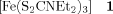Journal list menu
Export Citations
Download PDFs
Cover Picture (Angew. Chem. Int. Ed. Engl. 14/1994)
- First Published: August 2, 1994
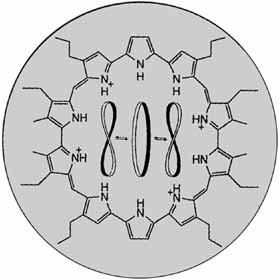
The cover picture shows the chemical formula of the tetraprotonated form of turcasarin, to date the largest expanded porphyrin. This class of compounds includes both macrocyclic porphyrin analogues and ring systems with more than four pyrrole units. The 40 π-electron annulene turcasarin exists as a pair of enantiomers in solution and in the solid state; the individual enantiomers adopt a nearly C2-symmetric, twisted “figure-eight” conformation. The interconversion of the two conformers via an open “circular” conformation (shown in the center of the picture) is slow on the NMR timescale at room temperature. More about this cyclic oligopyrrole, which in the tetraprotonated form, in analogy to several other expanded porphyrins, could serve as an anion binding and transport agent, is reported by J. L. Sessleret al. on p. 1509 ff.
Graphical Abstract (Angew. Chem. Int. Ed. Engl. 14/1994)
- Pages: 1407-1414
- First Published: August 2, 1994
Reviews
Peptidyl-Prolyl cis/trans Isomerases and Their Effectors†
- Pages: 1415-1436
- First Published: August 2, 1994
The profound biological consequences of simple cis/trans isomerizations in the peptide chain and the complexity of the processes on the molecular level are evident from investigations of peptidyl-prolyl isomerases (PPIases), which have been known for only a few years. Two families of proteins among the PPIases have been identified along with several inhibitors and binding proteins. But much remains to be discovered.
Conformational Requirements for Sweet-Tasting Peptides and Peptidomimetics
- Pages: 1437-1451
- First Published: August 2, 1994
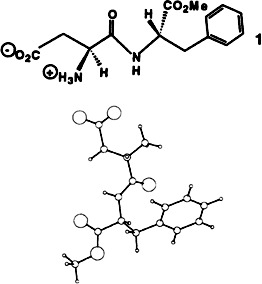
An L-shaped conformation is responsible for the sweet taste of aspartame (1) and other dipeptides, according to NMR studies and computer simulations of 1 and twelve analogues. Since the conformations of the compounds are dominated by packing forces in the solid state, the X-ray crystal structures do not reflect the bioactive conformations.
Highlights
Reactions in Supercritical Carbon Dioxide
- Pages: 1452-1455
- First Published: August 2, 1994
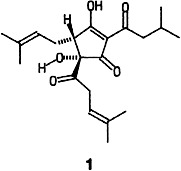
An efficient method for producing tasty beer: characteristic bittering agents such as trans-isohumulone (1) can be obtained readily by photolysis of the hops extract in supercritical CO2 in an established industrial process without large losses incurred on boiling. Nontoxic, environmentally friendly, cheap, nonflammable, and applicable on a large scale—these assets of supercritical CO2 have long been recognized for extractions; this highlight shows that supercritical CO2 is also advantageous for many reactions.
Triple Bonds in Small Rings: Testing the Limits of Chemical Bonds
- Pages: 1455-1456
- First Published: August 2, 1994
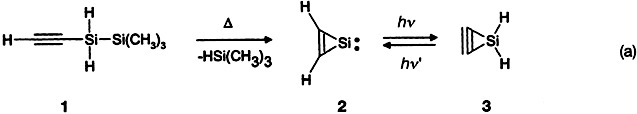
Mission Impossible? This is hardly true for the quest for small-ring alkynes. Maier et al. (Angew. Chem. Int. Ed. Engl. 1994, 33, 1248) combined a new method of gas-phase pyrolysis with matrix isolation techniques to synthesize a three-membered ring compound with a CC bond, the CH2Si isomer 3, from disilane 1 [Eq. (a)] and characterize it by spectroscopy. More information on the exact bonding and the anticipated reactivity of this highly strained compound are eagerly awaited.
Editorial
Enantioselective Reactions in a Static Magnetic Field-A False Alarm!
- Page: 1457
- First Published: August 2, 1994
Too good to be true—in hindsight unfortunately this must be said about a publication by G. Zadel et al. that appeared in the second February issue of Angewandte Chemie entitled “Enantioselective Reactions in a Static Magnetic Field”. The history of the publication and, in particular, the factors influencing the decision to publish are outlined. Regrettably, unnecessary time and effort were invested by many research groups worldwide in trying to reproduce these results. It is reassuring to know, however, that important publications are checked critically and very rapidly. In this particular case, established theories continue to hold true.
Correspondences
Attempts to Carry Out Enantioselective Reactions in a Static Magnetic Field
- Pages: 1458-1459
- First Published: August 2, 1994
They tried everything, but it still didn't work. Two research groups report here on their attempts to reproduce the results reported by Breitmaier et al. (Angew. Chem. Int. Ed. Engl. 1994, 33, 454). According to the subsequent statement by E. Breitmaier (see p. 1414 this issue), the claim of absolute asymmetric synthesis in a static homogeneous magnetic field is fraudulent.
Absolute Asymmetric Synthesis Solely under the Influence of a Static Homogeneous Magnetic Field?
- Pages: 1459-1461
- First Published: August 2, 1994
They tried everything, but it still didn't work. Two research groups report here on their attempts to reproduce the results reported by Breitmaier et al. (Angew. Chem. Int. Ed. Engl. 1994, 33, 454). According to the subsequent statement by E. Breitmaier (see p. 1414 this issue), the claim of absolute asymmetric synthesis in a static homogeneous magnetic field is fraudulent.
Bond-Stretch Isomers and Spin-State Isomers: A Comment on the Article “Bond-Stretch Isomers: Fact not Fiction”†
- Page: 1462
- First Published: August 2, 1994
What exactly is meant by bond-stretch isomerism? Confusion seems to reign about the phenomenon, partly because the original papers did not explicitly define the term. However, if limited to its original meaning, no bond-stretch isomers have yet been discovered, in spite of a recent claim to the contrary (see Correspondence by P. Giitlich, H. A. Goodwin, and D. N. Hendrickson in the second February issue of Angewandte Chemie).
Communications
Synthesis and Properties of a Vertically Stacked Porphyrin-Quinone(1)-Quinone(2) Cyclophane†
- Pages: 1463-1466
- First Published: August 2, 1994
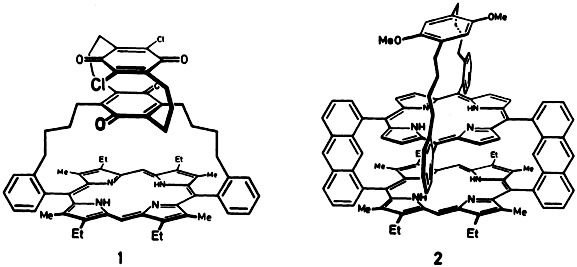
Model compounds for the study of the primary processes in photosynthesis continue to spur chemists on to elaborate syntheses. Latest efforts have resulted in the triple-decker compounds (“triads”) 1 and 2, in which two quinone and one porphyrin, and one quinone and two porphyrin units, respectively, are linked in rigid arrangements.
Synthesis and Properties of a Vertically Stacked Porphyrin(1)-Quinone(2)-Quinone Cyclophane†
- Pages: 1466-1468
- First Published: August 2, 1994
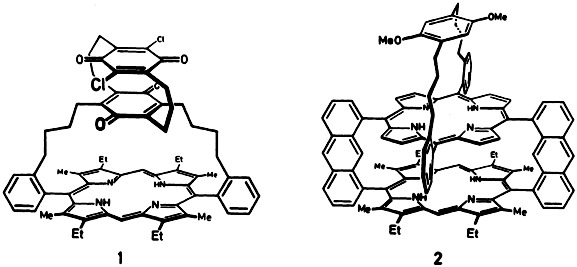
Model compounds for the study of the primary processes in photosynthesis continue to spur chemists on to elaborate syntheses. Latest efforts have resulted in the triple-decker compounds (“triads”) 1 and 2, in which two quinone and one porphyrin, and one quinone and two porphyrin units, respectively, are linked in rigid arrangements.
A Highly Convergent Synthesis of the Lewisy Blood Group Determinant in Conjugatable Form†‡
- Pages: 1468-1470
- First Published: August 2, 1994
The versatility of glycals both as glycosyl donors and as glycosyl acceptors is used in the synthesis of the Lewisy determinant. This and the oligosaccharide described in the following communication consist of a determinant, a carbohydrate spacer, and a group suitable for the conjugation to a carrier protein. The spacer present in these new glycoproteins minimizes the likelihood that the protein carrier will distort the recognition property of the determinant.
An Interactive Strategy for the Assembly of Complex, Branched Oligosaccharide Domains on a Solid Support: A Concise Synthesis of the Lewisb Domain in Bioconjugatable Form†
- Pages: 1470-1473
- First Published: August 2, 1994
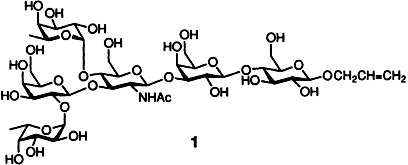
Minimal use of protecting groups is required in the synthesis of the branched hexa-saccharide Lewisb 1. Thus, donor and acceptor glycosyl units were linked stereo-selectively to the polymer-bound glycal in such a way that an oligosaccharide recognition element is formed which can be bound to a carrier protein.
The First Mononuclear Nitrosyl(oxo)molybdenum Complex: Side-On Bonded and μ3-Bridging NO Ligands in [{MoL(NO)(O)(OH)}2]NaPF6·H2O†
- Pages: 1473-1476
- First Published: August 2, 1994
![The First Mononuclear Nitrosyl(oxo)molybdenum Complex: Side-On Bonded and μ3-Bridging NO Ligands in [{MoL(NO)(O)(OH)}2]NaPF6·H2O](/cms/asset/53549b0c-de01-400f-bf2c-5add137f4a34/must001.jpg)
Linked through sodium ions to form a tetranuclear complex, the neutral unit [MoL(NO)(O)(OH)] of the title compound displays two types of bonding between the Na+ ions and NO (shown on the right) in the crystalline state. The bonding in this and similar monomeric nitrosyloxo complexes can be described by a synergistic bonding model. L = 1,4,7-triiso-propyl-1,4,7-triazacyclononane.
On the Helicity of Oligomeric Formaldehyde
- Pages: 1476-1478
- First Published: August 2, 1994
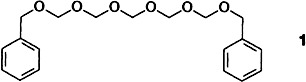
Even this simple helical molecule, the benzyl-protected pentamer 1, has a few surprises in store. Two independent conformers of 1 with the same absolute configuration are found in the unit cell which demonstrate an unprecedented form of isomerism. The helicity of the oligomeric formaldehyde is definitely a consequence of stereoelectronic effects.
Synthesis and Structure of the First [22]Metallocenophanes†‡
- Pages: 1479-1480
- First Published: August 2, 1994
![Synthesis and Structure of the First [22]Metallocenophanes](/cms/asset/741af324-a436-43bd-9c9d-20d6801282f6/must001.jpg)
The dark red Fe and colorless ZrCl2 complexes 2 are easily accessible from the doubly bridged biscyclopentadienes 1. The substantial torsion of the two eclipsing decks of the conformationally rigid ligand framework of 2 exposes the metal center and points to the potential of such complexes as catalysts for the stereoselective polymerization of alkenes.
Heterodimetal–Betaine Chemistry: Catalytic and Stoichiometric Coupling of Alkynyl Ligands under the Joint Influence of Zirconium and Boron Centers†
- Pages: 1480-1482
- First Published: August 2, 1994
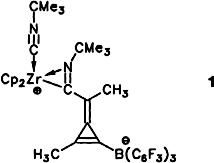
Readily accessible from bis(propynyl)zirconocene, the organometallic betaines like 1 form by an intramolecular alkyne insertion and trapping reaction with tBuNC from an intermediate that has a hexadiyne ligand unsymmetrically coordinated to the Zr and B centers. This diyne complex is obtained in a rapid CC coupling reaction reaction between the starting material and B(C6F5)3.
Chlorine Trioxide: Spectroscopic Properties, Molecular Structure, and Photochemical Behavior†
- Pages: 1482-1484
- First Published: August 2, 1994
Though postulated for years, now detected for the first time! The symmetric ClO3 radical was synthesized by vacuum flash pyrolysis of chlorine perchlorate and trapped by matrix isolation for UV and IR spectroscopic studies. The OClO bond angle is 113.5±2° and the ClO bond length 150.0±1 pm. The photolysis of ClO3 yields the unsymmetric isomer OClOO.
(tBuSi)2(PC6H11)3: A Propellane-Like Cyclosilaphosphane with Remarkable Structure†‡
- Pages: 1484-1487
- First Published: August 2, 1994
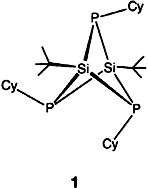
An unusually short SiSi distance of 2.518(3) Å despite an antibonding interaction between these centers and a small endocyclic angle of 67° at phosphorus, these are the surprising structural features of the title compound 1, which forms by cyclocondensation of tBuSiFCl2 with CyPHLi in 33% yield. The structure of 1 reflects a tug-of-war between the three SiPSi and the six PSiP angles, Cy = cyclohexyl.
A Highly Distorted and an Undistorted Borirane, CBH Hyperconjugation Induced by CSiH Hyperconjugation†
- Pages: 1487-1489
- First Published: August 2, 1994
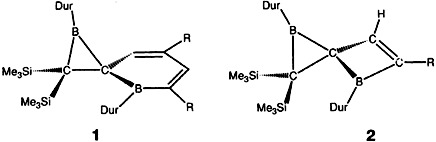
Hyperconjugation is the reason for the different structures of 1 and 2: in 1 the electron deficiency of the boracyclohexadiene leads to strong hyperconjugation with the CB bond in the spiro-linked three-membered ring, and between the thus more positive boron atom and a CSi bond. In 2, in contrast, the electron deficiency of the boron atom of the four-membered ring is reduced by formation of a 2π electron homoarene by using the n electrons of the CC double bond. Dur = 2,3,5,6-tetramethylphenyl, R = SiMe3.
Stable Tetrakis(trialkylsilyl)disilenes; Synthesis, X-Ray Structures, and UV/VIS Spectra†
- Pages: 1489-1491
- First Published: August 2, 1994
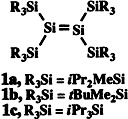
Significant downfield shifts in the NMR spectrum are displayed by the central Si atoms in compounds 1 relative to those of disilenes not having four silyl substituents. The extraordinary color change of 1c on dissolving in hexane (yellow → deep red) is particularly impressive and indicates a conformational change caused by reduction in steric strain.
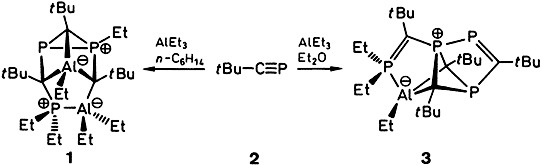
The First Carbon–Phosphorus–Aluminum Cage Compounds: Cyclooligomerization of Phosphaalkynes with Trialkylaluminum Compounds†‡
- Pages: 1491-1493
- First Published: August 2, 1994
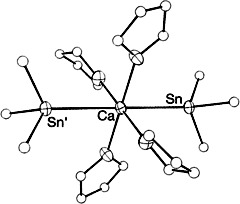
Synthesis and Molecular Structure of Calcium Bis(trimethylstannanide)·4THF†
- Pages: 1493-1495
- First Published: August 2, 1994
Keronopsins A and B, Chemical Defence Substances of the Marine Ciliate Pseudokeronopsis rubra (Protozoa): Identification by Ex Vivo HPLC
- Pages: 1495-1497
- First Published: August 2, 1994
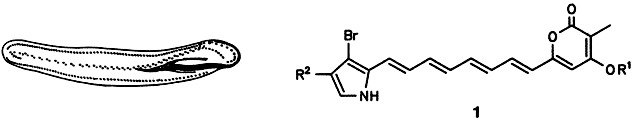
What happens when protozoa are injected onto an HPLC column? Because they do not have a rigid cell wall, they instantaneously release their contents, which can thus be analyzed without loss or opportunity for artefact formation (“ex vivo HPLC”). This method applied to the example, Pseudokeronopsis rubra (picture below), showed that the organism stores 1 in the form of the sulfate ester (R1 = SO3Na), which only on destruction of the cells is converted enzymatically into the more toxic pyrone 1 (R1 = H), R2 = H, Br.
Single-Crystal EPR Spectroscopy on [57Fe(NO)(S2CNEt2)2]: The “Triplet Signal” in the EPR Spectrum of [Fe(S2CNEt2)3]†
- Pages: 1497-1499
- First Published: August 2, 1994
![Single-Crystal EPR Spectroscopy on [57Fe(NO)(S2CNEt2)2]: The “Triplet Signal” in the EPR Spectrum of [Fe(S2CNEt2)3]](/cms/asset/cb628877-8396-4150-9cb9-b2cec0fc9123/must001.jpg)
Traces of nitrate in the starting material FeCl3 lead to the formation of the analogous nitrosyliron(I) complex 2 during the synthesis of the tris(dithiocarbamato)iron complex 1. EPR studies on 57Fe-labeled 2 show that this complex gives rise to the triplet signal in the EPR spin-crossover studies on 1 at g = 2.0 that has been discussed for a long time.
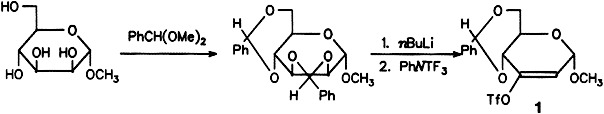
Enol Triflate Pyranoses, Versatile Reagents for the Formation of Conjugated Systems on Pyranoses†
- Pages: 1499-1501
- First Published: August 2, 1994
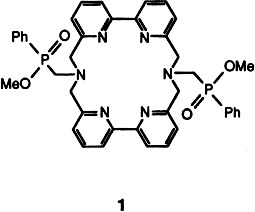
Luminescent Eu3+ and Tb3+ Complexes of a Branched Macrocyclic Ligand Incorporating 2,2′-Bipyridine in the Macrocycle and Phosphinate Esters in the Side Arms†
- Pages: 1501-1503
- First Published: August 2, 1994
A New Class of Novel Macrocyclic Mesogens†
- Pages: 1503-1506
- First Published: August 2, 1994
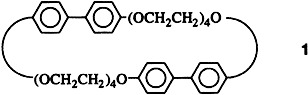
The clearing temperature is unexpectedly higher for the liquid crystalline phases of the macrocycle 1 (209°C) than for comparable acyclic biphenyls. The coupling of two appropriate rigid units through flexible terminal chains is apparently a highly effective method of inducing and stabilizing the mesophase.
1,3-Dialkoxycalix[4]arenecrowns-6 in 1,3-Alternate Conformation: Cesium-Selective Ligands that Exploit Cation-Arene Interactions†
- Pages: 1506-1509
- First Published: August 2, 1994
![1,3-Dialkoxycalix[4]arenecrowns-6 in 1,3-Alternate Conformation: Cesium-Selective Ligands that Exploit Cation-Arene Interactions](/cms/asset/1caad0ea-b527-4d3f-95f2-7fd2d375dd07/must001.jpg)
Exceptionally high selectivity for cesium ions (α(Cs/Na) > 33000) is displayed by calix[4]arenecrowns-6 in the 1,3-alternate conformation. They also remove 137Cs quantitatively (>96%) from radioactive waste that is 1 M in HNO3. The complexation properties result from the simultaneous operation of several effects: the size of the crown ether ring, the polarity of the calix conformation, and the strength of the cation/π-electron interactions. The latter interaction was evident in the X-ray crystal structure of the cesium complex (shown on the right).
Turcasarin, the Largest Expanded Porphyrin to Date†‡
- Pages: 1509-1512
- First Published: August 2, 1994
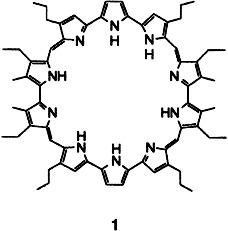
40π-Electrons and ten pyrrole units are found in macrocycle 1, the largest expanded porphyrin reported thus far. The tetrahydrochloride salt of 1 was characterized in solution and in the solid state. These investigations indicate that the macrocycle exists as a pair of enantiomers whose chirality arises from a twist of the ring.
Nonreactive Interactions between Ethene and Halogens: Detection of a π-Donor Complex C2H4BrCl by Rotational Spectroscopy†
- Pages: 1512-1513
- First Published: August 2, 1994
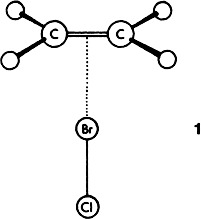
The outer complex initially formed on electrophilic addition has been shown to have the structure 1 in the prototypical reaction of ethene with BrCl. The rotational spectra of two isotopomers of a weakly bound complex were observed by Fourier transform microwave spectroscopy with a fast-mixing nozzle. This result is important for the understanding of the mechanism of electrophilic addition, a fundamental reaction in organic chemistry.
Stable Flexible Fibers and Rigid Tubules Made from Single-Chain Perfluoroalkylated Amphiphiles†
- Pages: 1514-1515
- First Published: August 2, 1994
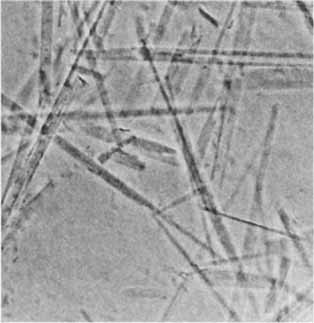
Up to 50 um long, stable, cylindrical aggregates (shown on the right) are formed spontaneously from dispersions of perfluoroalkylated, single-chain, nonchiral amphiphiles derived from dimorpho-linophosphoramidate and phosphocholine. Depending on the polar head group, rigid hollow tubules or thinner, longer, flexible fibers are obtained. Thus, the strong hydrophobic effect of the fluorinated chains alone appears to be sufficient to promote the formation of assemblies more stable than vesicles.
Metal Complexes of Marine Peptide Metabolites: A Novel Ag4 Cluster†
- Pages: 1516-1518
- First Published: August 2, 1994
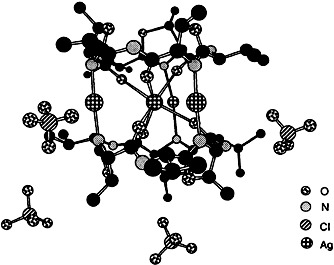
Silver(I) ions are bound selectively and with high cooperativity to the peptide westiellamide obtained from Lisso-clinum. The complex contains a unique [Ag4]4+ cluster sandwiched between two neutral macrocyclic ligands (see structure on the right). The importance of metal chelation for the biological activity is currently under investigation.
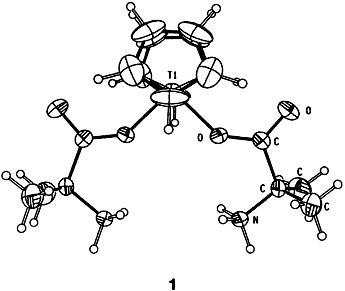
Synthesis and Structure of the First Titanium(IV) α-Amino Acid Complexes†
- Pages: 1518-1519
- First Published: August 2, 1994
Book Reviews
Book Review: Probability, Fractals and the Physical World: Chaos and Complexity. By B. Kaye
- Page: 1521
- First Published: August 2, 1994
New Books
New Books (Angew. Chem. Int. Ed. Engl. 14/1994)
- Page: 1522
- First Published: August 2, 1994