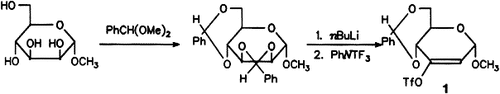
Enol Triflate Pyranoses, Versatile Reagents for the Formation of Conjugated Systems on Pyranoses†
Dr. Yousef Al-Abed
Abteilung für Physikalische Biochemie des Physiologisch-chemischen Instituts der Universität Hoppe-Seyler-Strasse 4, D-72076 Tübingen (FRG) Telefax: Int. code + (7071)29-3361
Search for more papers by this authorTaleb H. Al-Tel
Abteilung für Physikalische Biochemie des Physiologisch-chemischen Instituts der Universität Hoppe-Seyler-Strasse 4, D-72076 Tübingen (FRG) Telefax: Int. code + (7071)29-3361
Search for more papers by this authorChristoph Schröder
Abteilung für Physikalische Biochemie des Physiologisch-chemischen Instituts der Universität Hoppe-Seyler-Strasse 4, D-72076 Tübingen (FRG) Telefax: Int. code + (7071)29-3361
Search for more papers by this authorCorresponding Author
Prof. Dr. Wolfgang Voelter
Abteilung für Physikalische Biochemie des Physiologisch-chemischen Instituts der Universität Hoppe-Seyler-Strasse 4, D-72076 Tübingen (FRG) Telefax: Int. code + (7071)29-3361
Abteilung für Physikalische Biochemie des Physiologisch-chemischen Instituts der Universität Hoppe-Seyler-Strasse 4, D-72076 Tübingen (FRG) Telefax: Int. code + (7071)29-3361Search for more papers by this authorDr. Yousef Al-Abed
Abteilung für Physikalische Biochemie des Physiologisch-chemischen Instituts der Universität Hoppe-Seyler-Strasse 4, D-72076 Tübingen (FRG) Telefax: Int. code + (7071)29-3361
Search for more papers by this authorTaleb H. Al-Tel
Abteilung für Physikalische Biochemie des Physiologisch-chemischen Instituts der Universität Hoppe-Seyler-Strasse 4, D-72076 Tübingen (FRG) Telefax: Int. code + (7071)29-3361
Search for more papers by this authorChristoph Schröder
Abteilung für Physikalische Biochemie des Physiologisch-chemischen Instituts der Universität Hoppe-Seyler-Strasse 4, D-72076 Tübingen (FRG) Telefax: Int. code + (7071)29-3361
Search for more papers by this authorCorresponding Author
Prof. Dr. Wolfgang Voelter
Abteilung für Physikalische Biochemie des Physiologisch-chemischen Instituts der Universität Hoppe-Seyler-Strasse 4, D-72076 Tübingen (FRG) Telefax: Int. code + (7071)29-3361
Abteilung für Physikalische Biochemie des Physiologisch-chemischen Instituts der Universität Hoppe-Seyler-Strasse 4, D-72076 Tübingen (FRG) Telefax: Int. code + (7071)29-3361Search for more papers by this authorThis work was supported by the Fonds der Chemischen Industrie and by the Deutschen Akademischen Austauschdienst (doctoral stipendia for Y. A.-A. and T. H. A.-T.)
Graphical Abstract
References
- 1(a) J. C. López, E. Lameignère, C. Burnouf, A. A. Laborde, A. A. Ghini, A. Olesker, G. Lukacs, Tetrahedron 1993, 49, 7701–7722; (b) R. M. Giuliano, A. D. Jordan, Jr., A. D. Gauthier, K. Hoogsteen, J. Org. Chem. 1993, 58, 4979–4988; (c) R. Tsang, B. Eraser-Reid, J. Org. Chem. 1992, 57, 1065–1067; (d) K. S. Kim, I. H. Cho, Y. H. Joo, I. Y. Yoo, J. H. Song, J. H. Ko, Tetrahedron Lett. 1992, 33, 4029–4032; (e) J. Herscovici, S. Delatre, K. Antonakis, Tetrahedron Lett. 1991, 32, 1183–1186; (f) R. M. Giuliano, J. H. Buzby, N. Macropulos, J. P. Springer, J. Org. Chem. 1990, 55, 3555–3562; (g) C. Burnouf, J. C. López, F. G. Calvo-Flores, M. A. Laborde, A. Olesker, G. Lukacs, J. Chem. Soc. Chem. Commun. 1990, 823–825; (h) A. Mukhopadhyay, S. M. Ali, M. Husain, S. N. Suryawanshi, D. S. Bhakuni, Tetrahedron Lett. 1989, 30, 1853–1856; (i) J. C. López, E. Lameignere, G. Lukacs, J. Chem. Soc. Chem. Commun. 1988, 514–515; (j) B. H. Lipshutz, S. L. Nguyen, T. R. Elworthy, Tetrahedron 1988, 44, 3355–3364; (k) J. C. López, E. Lameignere, G. Lukacs, J. Chem. Soc. Chem. Commun. 1988, 706–707; (l) Y. Chapleur, M.-N. Euvard, Biologische Anstalt Helgoland, Jahresbericht 1987, 884–885; (m) M. Brehm, W. G. Dauben, P. Köhler, F. W. Lichtenthaler, Angew. Chem. 1987, 99, 1318–1319; Angew. Chem. Int. Ed. Engl. 1987, 26, 1271–1273; (n) R. M. Giuliano, J. H. Buzby, Carbohydr. Res. 1986, 158, c1–c4.
- 2 D. Morton, W. Weckerle, Carbohydr. Res. 1975, 44, 227–240.
- 3 J. E. McMurry, W. J. Scott, Tetrahedron Lett. 1983, 24, 979–982.
- 4 K. Kondo, M. Sodeoka, M. Mori, M. Shibasaki, Synthesis 1993, 920–930; (b) K. Ritter, Synthesis 1993, 735 762; (c) T. N. Mitchell, Synthesis 1992, 803–815; (d) V. N. Kalinin, Synthesis 1992, 413–432; W. J. Scott, J. E. McMurry, Acc. Chem. Res. 1988, 21, 47–54.
- 5 W. J. Scott, J. K. Stille, J. Am. Chem. Soc. 1986, 108, 3033–3040.
- 6 D. Seyferth, S. C. Vick, J. Organomet. Chem. 1978, 144, 1–12.
- 7 A. J. Leusink, H. A. Budding, J. W. Marsman, J. Organomet. Chem. 1967, 9, 285–294.
- 8(a) A. S. Gybin, W. A. Smit, R. Caple, A. L. Veretenov, A. S. Shashkov, L. G. Vorontsova, M. G. Kurella, V. S. Chertkov, A. A. Carapetyan, A. Y. Kosnikov, M. S. Alexanyan, S. V. Lindeman, V. N. Panov, A. V. Maleev, Y. T. Struchkov, S. M. Sharpe, J. Am. Chem. Soc. 1992, 114, 5555–5566; (b) G. S. Mikaelian, A. S. Gybin, W. A. Smit, R. Caple, Tetrahedron Lett. 1985, 26, 12691272; (c) A. A. Schegolev, W. A. Smit, Y. B. Kalyan, M. Z. Krimer, R. Caple, Tetrahedron Lett. 1982, 23, 4419–4422.
- 9 W. J. Scott, M. R. Pena, K. Sward, S. J. Stoessel, J. K. Stille, J. Org. Chem. 1985, 50, 2303–2308.
- 10 G. T. Crisp, W. J. Scott, J. K. Stille, J. Am. Chem. Soc. 1984, 106, 7500–7506.
- 11 E. D. Bergman, D. Ginsburg, R. Pappo, Org. React. 1959, 10, 179–555.
- 12(a) S. E. Denmark, K. L. Habermas, G. A. Kite, T. K. Jones, Tetrahedron 1986, 42, 2821–2829; (b) M. Ramaiah, Synthesis 1984, 529–570; C. Santelli-Rouvier, M. Santelli, Synthesis 1983, 429–442.