Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture: Multiplexing Sensory Molecules Map Protons Near Micellar Membranes (Angew. Chem. Int. Ed. 25/2008)
- Page: 4609
- First Published: 03 June 2008

Like mapping other worlds In their Communication on page 4667 ff., A. P. de Silva et al. describe proton concentration maps of detergent micelles obtained by the fluorescent multiplexing sensors (space-filling model). These sensors can change their positions and report information comprised of pKa values and emission wavelengths. These sensors serve as nanosized robot vehicles, as they send detailed data from humanly inaccessible environments.
Inside Cover
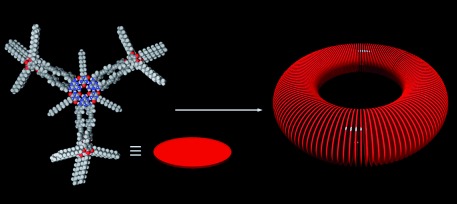
Inside Cover: Toroidal Nanoobjects from Rosette Assemblies of Melamine-Linked Oligo(p-phenyleneethynylene)s and Cyanurates (Angew. Chem. Int. Ed. 25/2008)
- Page: 4610
- First Published: 03 June 2008

Nanodonuts form from hierarchical self-organization of a melamine featuring π-conjugated oligo(para-phenyleneethynylene)s with a cyanurate. The toroidal nanostructures, with a diameter of 40 nm (see AFM image), form in decane under dilute conditions. S. Yagai, A. Ajayaghosh et al. propose in their Communication on page 4691 ff. that a hydrogen-bonded rosette is an essential intermediate supramolecular species for such a hierarchical self-organization process. The background picture shows a “bubble ring” made by a scuba diver.
Graphical Abstract
News
Spotlights on our sister journals: Angew. Chem. Int. Ed. 25/2008
- Pages: 4624-4625
- First Published: 03 June 2008
Inorganic Chemistry: G. Férey Awarded / Organometallic Chemistry: P. Knochel Honored / Materials Science: Prize to T. Mallouk
- Page: 4627
- First Published: 03 June 2008
Book Review
Practical Guide to Interpretive Near-Infrared Spectroscopy. By Jerry Workman, Jr. and Lois Weyer.
- Pages: 4628-4629
- First Published: 03 June 2008
Highlights
Organocatalytic Reactions with Acetaldehyde†
- Pages: 4632-4634
- First Published: 03 June 2008
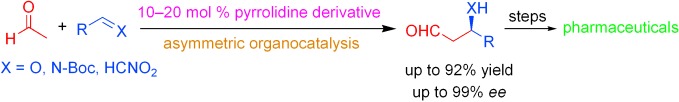
Big step, small molecule: Although acetaldehyde is the simplest of enolizable carbonyl compounds, and low-molecular-weight enamine/iminium-ion organocatalysis is the more elegant and most economical way to introduce chirality into a molecule, the first reports of the direct catalytic asymmetric reactions of acetaldehyde with electrophiles have just recently appeared (see scheme; Boc=tert-butyloxycarbonyl). Elaboration of the obtained adducts provides precursors for the synthesis of pharmaceuticals.
Postsynthetic Covalent Modification of Metal–Organic Framework (MOF) Materials
- Pages: 4635-4637
- First Published: 03 June 2008
Review
Asymmetric Organocatalysis: From Infancy to Adolescence
- Pages: 4638-4660
- First Published: 03 June 2008

As Bernini's David, in the prime of adolescence, fought Goliath armed only with a sling and a stone, organocatalysis uses simple organic molecules to address some fundamental issues of organic synthesis. Important questions concern the chemical efficiency of such processes and the development of viable and simple routes to natural products and drug candidates.
Communications
Supramolecular Capsules with Gated Pores from an Amphiphilic Rod Assembly†
- Pages: 4662-4666
- First Published: 03 June 2008
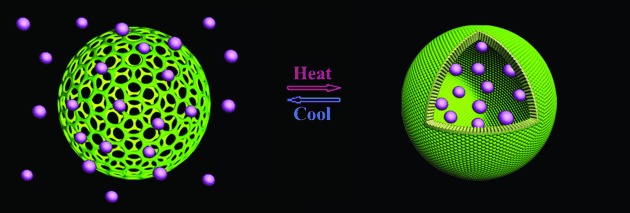
Please release me: Dumbbell-shaped rod amphiphiles self-assemble into hollow spheres (see picture) with gated nanopores in the shell. These pores undergo reversible transitions from the open state to the closed state upon heating, and are capable of entrapping the cargos with preservation of their hollow spherical structure, in a manner similar to viral capsids.
Multiplexing Sensory Molecules Map Protons Near Micellar Membranes†
- Pages: 4667-4669
- First Published: 03 June 2008
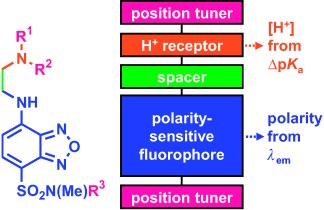
Hi-fi mapping: Multiplexing fluorescent sensors that simultaneously target proton concentration and polarity move to micellar nanospaces, self-regulate their positions, and report their pKa values and wavelengths, which are controlled by their local environments. Such sensory functions enable maps of proton gradients near micellar membranes to be drawn.
Chiral Bis(pyridylimino)isoindoles: A Highly Modular Class of Pincer Ligands for Enantioselective Catalysis†
- Pages: 4670-4674
- First Published: 03 June 2008
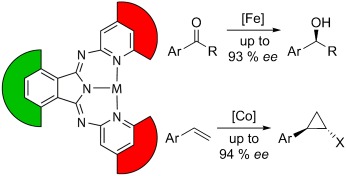
Protect your back: Chiral wedges (red, see scheme) at the wingtips of bis(2-pyridylimino)isoindole (bpi) pincer ligands with an appropriate protective hedge (green), to block the metal center from backside attack, in the backbone represent a new class of efficient 3d-metal catalysts. These catalysts gave excellent enantioselectivities in the iron-catalyzed hydrosilylation of arylketones and in the cobalt-catalyzed cyclopropanation of alkenes.
H2O2 Generation by Decamethylferrocene at a Liquid|Liquid Interface†
- Pages: 4675-4678
- First Published: 03 June 2008
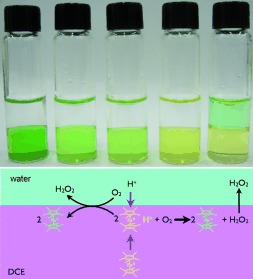
Hydrogen peroxide generation at a liquid|liquid interface occurs with a yield of 20 % with respect to the concentration of reducing agent (decamethylferrocene). The liquid|liquid interface supplies electrons from the reducing agent and protons from the aqueous phase to drive the reduction of O2 into H2O2, which is extracted into the aqueous phase during the course of reaction (see picture; DCE=1,2-dichloroethane).
Small-Molecule Inhibitors of Islet Amyloid Polypeptide Fibril Formation†
- Pages: 4679-4682
- First Published: 03 June 2008
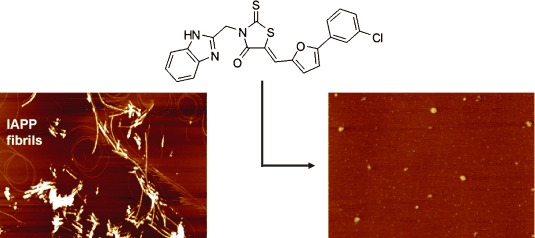
Small and effective: The pathological aggregation of amylin (IAPP), which leads to type II diabetes mellitus, is effectively inhibited by small-molecule rhodanine-based inhibitors at nanomolar concentrations. The prevention of aggregation by treatment with the inhibitor is demonstrated by AFM (see image).
Scaling Relationships for Adsorption Energies on Transition Metal Oxide, Sulfide, and Nitride Surfaces†
- Pages: 4683-4686
- First Published: 03 June 2008
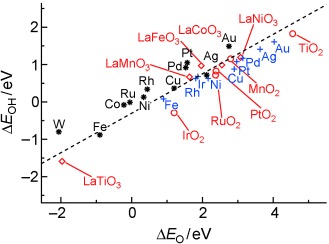
Getting on top of things: DFT calculations have been used to study the adsorption energies of O, OH, S, SH, N, NH, and NH2 on transition metal oxide, sulfide, and nitride surfaces. A scaling relationship was found between the adsorption energies of the intermediates and the adsorption energies of the atoms which is independent of the metal and depends only on the number of H atoms in the molecule (see graph).
Organocatalytic Asymmetric Synthesis of Versatile γ-Lactams†
- Pages: 4687-4690
- First Published: 03 June 2008
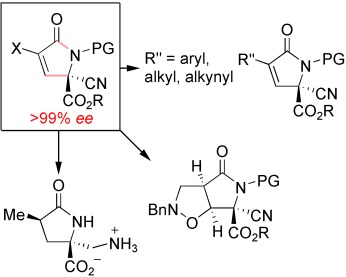
A good starting point: Optically pure γ-lactams have been prepared by organocatalytic enantioselective vinylic substitution. The parent lactam structure allows logical and systematic introduction of functionality around the periphery to access new derivatives of this important class of molecules. Bn=benzyl, PG=protecting group.
Toroidal Nanoobjects from Rosette Assemblies of Melamine-Linked Oligo(p-phenyleneethynylene)s and Cyanurates†
- Pages: 4691-4694
- First Published: 03 June 2008
A General and Efficient Method for the Suzuki–Miyaura Coupling of 2-Pyridyl Nucleophiles†
- Pages: 4695-4698
- First Published: 03 June 2008
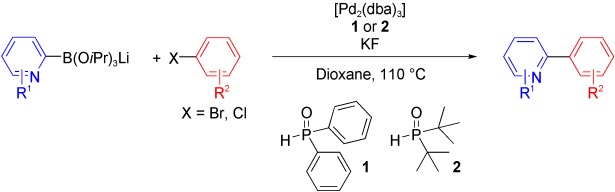
One of the most general systems for the cross-coupling of aryl and heteroaryl bromides and chlorides with 2-pyridyl-derived nucleophiles has been developed, for which catalysts comprising [Pd2(dba)3] and either diaryl (1) or dialkyl phosphine oxide (2) supporting ligands were found to be ideal (see scheme).
Tandem Modification of Metal–Organic Frameworks by a Postsynthetic Approach†
- Pages: 4699-4702
- First Published: 03 June 2008
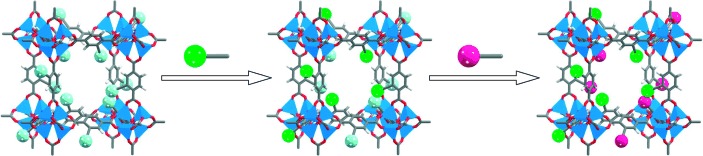
More than a one-trick pony: Postsynthetic modification of metal–organic frameworks (MOFs) permits stepwise, “tandem” modification of the lattice. An MOF bearing reactive functional groups can be subjected to a controllable, heterogeneous transformation sequence while retaining its structural integrity and microporosity. This approach allows the synthesis of MOFs bearing multiple functional groups (represented in the scheme by green and pink spheres).
A Study of BF3-Promoted ortho Lithiation of Anilines and DFT Calculations on the Role of Fluorine–Lithium Interactions†
- Pages: 4703-4706
- First Published: 03 June 2008
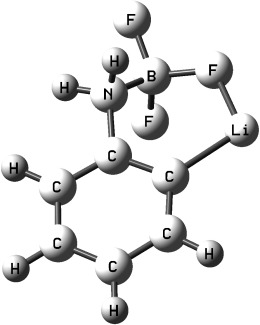
Director's cut: The poor directing ability of the dimethylamino group in directed ortho metalation is altered by complexation to BF3, which makes it more strongly activating than methoxy and chloro groups. DFT calculations of simplified lithiated intermediates reveal a small role of the inductive effect, but a distinctive F⋅⋅⋅Li interaction that leads to a F–Li distance which is remarkably similar to the C–Li distance.
From Native to Non-Native Two-Dimensional Protein Lattices through Underlying Hydrophilic/Hydrophobic Nanoprotrusions†
- Pages: 4707-4710
- First Published: 03 June 2008
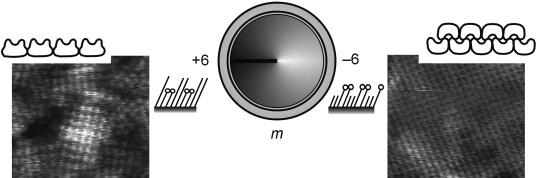
Tuning to the terminals: Controlled tuning of protein–substrate interactions induces a transition from native to non-native protein crystals (see AFM images) as—depending on m, the chain-length difference, which determines whether surface protrusions are hydroxy or methyl functionalized—the underlying non-ion-mediated interactions are gradually transformed from mainly associative (H-bonding) to hydrophobic.
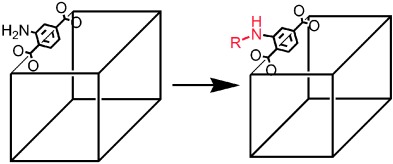
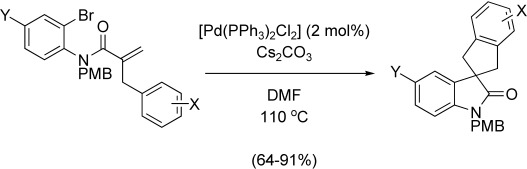
Palladium-Catalyzed Tandem Heck Reaction/CH Functionalization—Preparation of Spiro-Indane-Oxindoles
- Pages: 4711-4714
- First Published: 03 June 2008
Four-Electron Oxidative Formation of Aryl Diazenes Using a Tantalum Redox-Active Ligand Complex†
- Pages: 4715-4718
- First Published: 03 June 2008
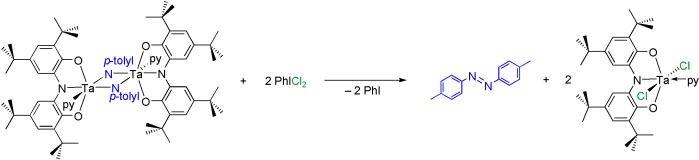
The active ingredient: The bis(μ-imido) tantalum dimer {[ONOred]Ta(μ-N-p-tolyl)(py)}2 releases the aryl diazene (p-tolyl)NN(p-tolyl) upon four-electron oxidation with PhICl2 (see scheme; py=pyridine). Studies suggest that the redox-active [ONOred]3− ligands act to collect single-electron oxidation equivalents for the multielectron reaction.
Catalytic Asymmetric Michael Reactions of Acetaldehyde†
- Pages: 4719-4721
- First Published: 03 June 2008
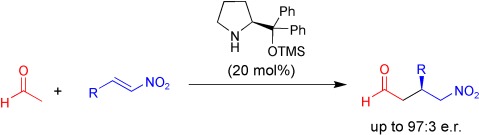
Acetaldehyde, now a big contender: A silyl prolinol derivative was found to catalyze the first Michael addition of acetaldehyde with both aromatic and aliphatic nitroolefins in excellent enantioselectivities (see scheme, TMS=trimethylsilyl). The utility of the reaction is illustrated in the synthesis of three current pharmaceuticals and in the synthesis of an enantiopure 3-monosubstituted pyrrolidine.
Asymmetric Michael Reaction of Acetaldehyde Catalyzed by Diphenylprolinol Silyl Ether†
- Pages: 4722-4724
- First Published: 03 June 2008
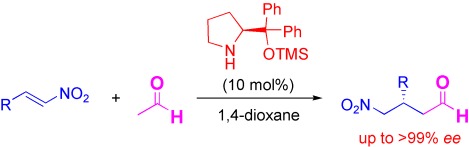
An acetaldehyde breakthrough: The catalytic asymmetric Michael addition reaction of acetaldehyde and various nitroalkenes in the presence of a chiral diphenylprolinol silyl ether organocatalyst is described (see scheme; TMS=trimethylsilyl). The desired 1,4-addition products, α-unsubstituted γ-nitro aldehydes, were obtained in good yields with excellent enantioselectivities.
Patterning the Surface of Colloidal Microspheres and Fabrication of Nonspherical Particles†
- Pages: 4725-4728
- First Published: 03 June 2008
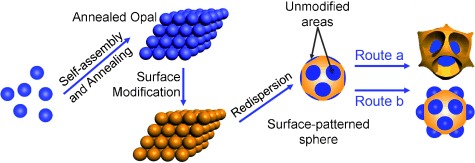
One seed for two: Microspheres with patterned surfaces were obtained by modifying the surface of microspheres of a self-assembled colloidal crystal and redispersion of the colloidal crystal into individual particles. With these surface-patterned spheres as seeds, two types of nonspherical particles were fabricated by the seeded particle growth method.
Ligand-Free Pd-Catalyzed Highly Selective Arylation of Allylic Esters with Retention of the Traditional Leaving Group†
- Pages: 4729-4732
- First Published: 03 June 2008
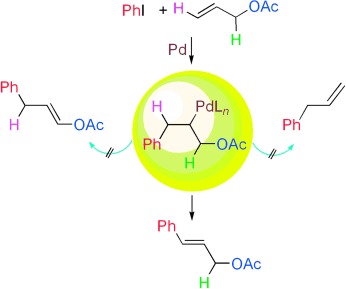
What leaving group? Organic halides and allylic esters undergo efficient Pd-catalyzed Heck reactions under mild conditions in air to form a new CC bond without elimination of the β-OAc group in the intermediate palladium complex. Instead a highly regioselective β-H elimination takes place to provide substituted derivatives of allylic alcohols (see scheme).
Palladium-Catalyzed Intermolecular Aerobic Oxidative Amination of Terminal Alkenes: Efficient Synthesis of Linear Allylamine Derivatives†
- Pages: 4733-4736
- First Published: 03 June 2008
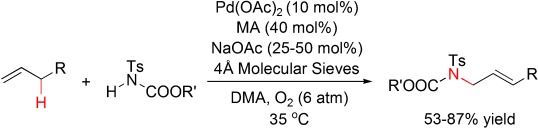
O2-coupled allylic CH amination: A first general palladium-mediated intermolecular aerobic oxidative allylic amination was developed to synthesize linear (E)-allylimides with high regioselectivity (see scheme; MA=maleic anhydride). The proposed mechanism involves an allylic CH activation with subsequent nitrogen nucleophile substitution. The catalytic system allows efficient dioxygen-coupled turnover without additional cocatalysts.
Total Synthesis of (+)-Neopeltolide†
- Pages: 4737-4739
- First Published: 03 June 2008
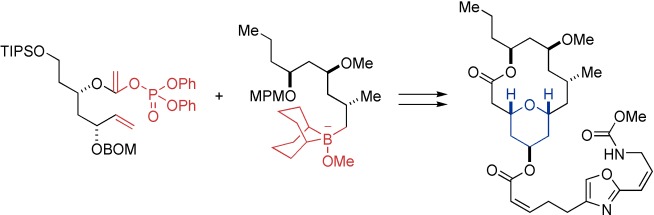
Rapid elaboration: (+)-Neopeltolide, a novel marine metabolite with potent cytotoxicity against several cancer cell lines, was the target of an efficient total synthesis (see scheme). The construction of the 2,4,6-trisubstituted tetrahydropyran substructure is based on a Suzuki–Miyaura coupling/ring-closing metathesis sequence. BOM=benzyloxymethyl, MPM=4-methoxyphenylmethyl, TIPS=triisopropylsilyl.
Stable Five- and Six-Coordinate Cobalt(III) Complexes with a Pentadentate Bispidine Ligand†
- Pages: 4740-4743
- First Published: 03 June 2008
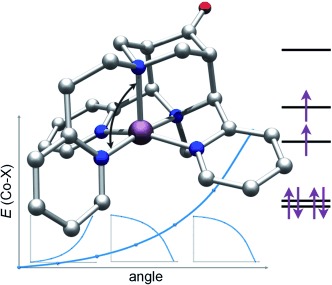
I am ligand: A pentadentate bispidine ligand with two tertiary amine and three pyridine donors stabilizes the uncommon intermediate-spin electronic configuration of CoIII (S=1; see picture: C gray, N blue, O red, Co violet). Dissociation of the monodentate coligand yields a catalytically active pentacoordinate complex.
Metal-Free Conversion of Methane and Cycloalkanes to Amines and Amides by Employing a Borylnitrene†
- Pages: 4744-4747
- First Published: 03 June 2008
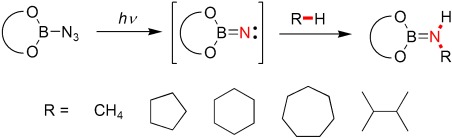
CH insertion: Borylnitrenes, which are generated in situ by photoylsis of azides, convert unactivated alkanes by intermolecular CH insertion into aminoboranes (see scheme), which in turn can be reacted further to give amines or amides. The boryl group serves two purposes: it converts the nitrene into a highly reactive BN vinylidene analogue, and it is easily cleaved from the product.
dsRNA-Functionalized Multifunctional γ-Fe2O3 Nanocrystals: A Tool for Targeting Cell Surface Receptors†
- Pages: 4748-4752
- First Published: 03 June 2008
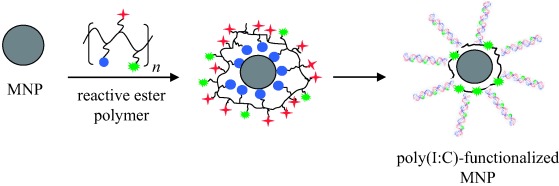
Specific cell recognition: Magnetic γ-Fe2O3 nanoparticles (MNPs) functionalized with a fluorescently labeled polymer and polyinosinic-polycytidylic acid [poly(I:C)] allow the specific visualizion of the TLR3 receptors on the the surface of Caki-1 cells. The expression of TLR3 on the Caki-1 cells was demonstrated independently by RT-PCR and immunostaining techniques.
Chromophore Heterogeneity and Photoconversion in Phytochrome Crystals and Solution Studied by Resonance Raman Spectroscopy†
- Pages: 4753-4755
- First Published: 03 June 2008
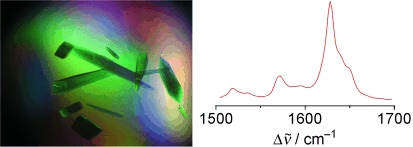
Crystals of the photoreceptor phytochrome were studied nondestructively by resonance Raman spectroscopy. The spectra of the chromophore-binding domains (CBD) of phytochrome from Deinococcus radiodurans, from which three-dimensional structures have been determined, demonstrate the same chromophore structure in the parent Pr state as in solution. Unlike CBD, crystals of phytochrome from Agrobacterium tumefaciens including the CBD and the PHY domain can be photoconverted to a Meta-Rc-like state.