Multiplexing Sensory Molecules Map Protons Near Micellar Membranes†
Seiichi Uchiyama Dr.
School of Chemistry and Chemical Engineering, Queen's University, Belfast BT9 5AG (Northern Ireland), Fax: (+44) 28-9097-4687
Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5841-4768
Search for more papers by this authorKaoru Iwai Prof. Dr.
Department of Chemistry, Faculty of Science, Nara Women's University, Kitauoya-Nishimachi, Nara 630-8506, Japan
Search for more papers by this authorA. Prasanna de Silva Prof. Dr.
School of Chemistry and Chemical Engineering, Queen's University, Belfast BT9 5AG (Northern Ireland), Fax: (+44) 28-9097-4687
Search for more papers by this authorSeiichi Uchiyama Dr.
School of Chemistry and Chemical Engineering, Queen's University, Belfast BT9 5AG (Northern Ireland), Fax: (+44) 28-9097-4687
Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5841-4768
Search for more papers by this authorKaoru Iwai Prof. Dr.
Department of Chemistry, Faculty of Science, Nara Women's University, Kitauoya-Nishimachi, Nara 630-8506, Japan
Search for more papers by this authorA. Prasanna de Silva Prof. Dr.
School of Chemistry and Chemical Engineering, Queen's University, Belfast BT9 5AG (Northern Ireland), Fax: (+44) 28-9097-4687
Search for more papers by this authorWe thank The Daiwa Anglo-Japanese Foundation, Invest NI (RTD COE 40) (Japan) Society for the promotion of Science, W. T. Silva and S. T. Herath for support and help.
Graphical Abstract
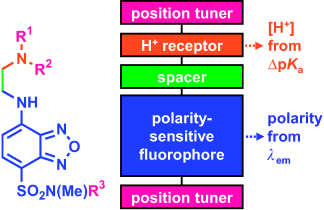
Hi-fi mapping: Multiplexing fluorescent sensors that simultaneously target proton concentration and polarity move to micellar nanospaces, self-regulate their positions, and report their pKa values and wavelengths, which are controlled by their local environments. Such sensory functions enable maps of proton gradients near micellar membranes to be drawn.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z801516_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher, T. E. Rice, Chem. Rev. 1997, 97, 1515–1566;
- 1bJ. F. Callan, A. P. de Silva, D. C. Magri, Tetrahedron 2005, 61, 8551–8588;
- 1cE. V. Anslyn, J. Org. Chem. 2007, 72, 687–699.
- 2
- 2aJ.-P. Sauvage, Acc. Chem. Res. 1998, 31, 611–619;
- 2bC. P. Collier, E. W. Wong, M. Belohradský, F. M. Raymo, J. F. Stoddart, P. J. Kuekes, R. S. Williams, J. R. Heath, Science 1999, 285, 391–394;
- 2cV. Amendola, L. Fabbrizzi, C. Mangano, P. Pallavicini, Acc. Chem. Res. 2001, 34, 488–493;
- 2dV. Balzani, A. Credi, M. Venturi, Molecular Devices and Machines, Wiley-VCH, Weinheim, 2003;
10.1002/3527601600 Google Scholar
- 2eV. Serreli, C.-F. Lee, E. R. Kay, D. A. Leigh, Nature 2007, 445, 523–527;
- 2fS. Kobatake, S. Takami, H. Muto, T. Ishikawa, M. Irie, Nature 2007, 446, 778–781.
- 3
- 3aA. P. de Silva, H. Q. N. Gunaratne, C. P. McCoy, Nature 1993, 364, 42–44;
- 3bA. P. de Silva, N. D. McClenaghan, Chem. Eur. J. 2004, 10, 574–586;
- 3cS. Uchiyama, N. Kawai, A. P. de Silva, K. Iwai, J. Am. Chem. Soc. 2004, 126, 3032–3033;
- 3dU. Pischel, Angew. Chem. 2007, 119, 4100–4115;
10.1002/ange.200603990 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4026–4040;
- 3eA. P. de Silva, S. Uchiyama, Nat. Nanotechnol. 2007, 2, 399–410.
- 4
- 4aM. Schmittel, H.-W. Lin, Angew. Chem. 2007, 119, 911–914;
10.1002/ange.200603362 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 893–896;
- 4bT. Suzuki, K. Ohta, T. Nehira, H. Higuchi, E. Ohta, H. Kawai, K. Fujiwara, Tetrahedron Lett. 2008, 49, 772–776.
- 5For related but different ideas, see:
- 5aM. D. P. de Costa, A. P. de Silva, S. T. Pathirana, Can. J. Chem. 1987, 65, 1416–1419;
- 5bK. R. A. S. Sandanayake, T. D. James, S. Shinkai, Chem. Lett. 1995, 503–504.
- 6J. H. Fendler, Membrane Mimetic Chemistry, Wiley, New York, 1983, pp. 6–47.
- 7F. M. Harold, The Vital Force—A Study of BioEnergetics, Freeman, New York, 1986.
- 8
- 8aM. S. Fernández, P. Fromherz, J. Phys. Chem. 1977, 81, 1755–1761;
- 8bC. J. Drummond, F. Grieser, T. W. Healy, J. Phys. Chem. 1988, 92, 2604–2613;
- 8cT. Pal, N. R. Jana, Langmuir 1996, 12, 3114–3121.
- 9
- 9aR. A. Bissell, A. J. Bryan, A. P. de Silva, C. P. McCoy, J. Chem. Soc. Chem. Commun. 1994, 405–407;
- 9bY. Singh, A. Gulyani, S. Bhattacharya, FEBS Lett. 2003, 541, 132–136.
- 10S. Uchiyama, Y. Matsumura, A. P. de Silva, K. Iwai, Anal. Chem. 2003, 75, 5926–5935.
- 11
- 11aE. Bardez, E. Monnier, B. Valeur, J. Phys. Chem. 1985, 89, 5031–5036;
- 11bE. C. C. Melo, S. M. B. Costa, A. L. Maçanita, H. Santos, J. Colloid Interface Sci. 1991, 141, 439–453;
- 11cW. H. Steel, R. A. Walker, Nature 2003, 424, 296–299.
- 12P. S. Goyal, S. V. G. Menon, B. A. Dasannacharya, P. Thiyagarajan, Phys. Rev. E 1995, 51, 2308–2315.
- 13B. Lorber, J. B. Bishop, L. J. DeLucas, Biochim. Biophys. Acta Biomembr. 1990, 1023, 254–265.
- 14V. K. Aswal, P. S. Goyal, Phys. Rev. E 2000, 61, 2947–2953.
- 15The ΔpKa values for 10–18 are determined by using the pKa values for 1–9 in water instead of those for 10–18 because of insolubility.
- 16A. P. de Silva, H. Q. N. Gunaratne, J.-L. Habib-Jiwan, C. P. McCoy, T. E. Rice, J.-P. Soumillion, Angew. Chem. 1995, 107, 1889–1891;
10.1002/ange.19951071618 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 1728–1731.
- 17In the linear relationships between the emission wavelength and the ε value of solvent, 1–9 are treated as model compounds of 10–18, as 10–18 are insoluble in water.
- 18The use of differently weighted averages of two ε values did not affect the relative pattern of ΔpKa–polarity diagrams.
- 19S. Uchiyama, T. Santa, K. Imai, J. Chem. Soc. Perkin Trans. 2 1999, 569–576.