Total Synthesis of (+)-Neopeltolide†
Haruhiko Fuwa Dr.
Laboratory of Biostructural Chemistry, Graduate School of Life Sciences, Tohoku University, 1-1 Tsutsumidori-amamiya, Aoba-ku, Sendai 981-8555, Japan, Fax: (+81) 22-717-8896
Search for more papers by this authorShinya Naito
Laboratory of Biostructural Chemistry, Graduate School of Life Sciences, Tohoku University, 1-1 Tsutsumidori-amamiya, Aoba-ku, Sendai 981-8555, Japan, Fax: (+81) 22-717-8896
Search for more papers by this authorTomomi Goto
Laboratory of Biostructural Chemistry, Graduate School of Life Sciences, Tohoku University, 1-1 Tsutsumidori-amamiya, Aoba-ku, Sendai 981-8555, Japan, Fax: (+81) 22-717-8896
Search for more papers by this authorMakoto Sasaki Prof. Dr.
Laboratory of Biostructural Chemistry, Graduate School of Life Sciences, Tohoku University, 1-1 Tsutsumidori-amamiya, Aoba-ku, Sendai 981-8555, Japan, Fax: (+81) 22-717-8896
Search for more papers by this authorHaruhiko Fuwa Dr.
Laboratory of Biostructural Chemistry, Graduate School of Life Sciences, Tohoku University, 1-1 Tsutsumidori-amamiya, Aoba-ku, Sendai 981-8555, Japan, Fax: (+81) 22-717-8896
Search for more papers by this authorShinya Naito
Laboratory of Biostructural Chemistry, Graduate School of Life Sciences, Tohoku University, 1-1 Tsutsumidori-amamiya, Aoba-ku, Sendai 981-8555, Japan, Fax: (+81) 22-717-8896
Search for more papers by this authorTomomi Goto
Laboratory of Biostructural Chemistry, Graduate School of Life Sciences, Tohoku University, 1-1 Tsutsumidori-amamiya, Aoba-ku, Sendai 981-8555, Japan, Fax: (+81) 22-717-8896
Search for more papers by this authorMakoto Sasaki Prof. Dr.
Laboratory of Biostructural Chemistry, Graduate School of Life Sciences, Tohoku University, 1-1 Tsutsumidori-amamiya, Aoba-ku, Sendai 981-8555, Japan, Fax: (+81) 22-717-8896
Search for more papers by this authorThis work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Sports, Culture, Science and Technology (Japan).
Graphical Abstract
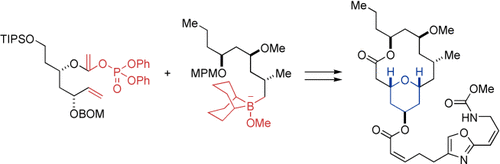
Rapid elaboration: (+)-Neopeltolide, a novel marine metabolite with potent cytotoxicity against several cancer cell lines, was the target of an efficient total synthesis (see scheme). The construction of the 2,4,6-trisubstituted tetrahydropyran substructure is based on a Suzuki–Miyaura coupling/ring-closing metathesis sequence. BOM=benzyloxymethyl, MPM=4-methoxyphenylmethyl, TIPS=triisopropylsilyl.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z801399_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. E. Wright, J. C. Botelho, E. Guzmán, D. Harmody, P. Linley, P. J. McCarthy, T. P. Pitts, S. A. Pomponi, J. K. Reed, J. Nat. Prod. 2007, 70, 412.
- 2W. Youngsaye, J. T. Lowe, F. Pohlki, P. Falifo, J. S. Panek, Angew. Chem. 2007, 119, 9371;
10.1002/ange.200704122 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 9211.
- 3D. W. Custer, T. P. Zabawa, K. A. Scheidt, J. Am. Chem. Soc. 2008, 130, 804.
- 4V. V. Vintonyak, M. E. Maier, Org. Lett. 2008, 10, 1239.
- 5S. K. Woo, M. S. Kwon, E. Lee, Angew. Chem. 2008, 120, 3286; Angew. Chem. Int. Ed. 2008, 47, 3242.
- 6For reviews of Suzuki–Miyaura coupling, see:
- 6aN. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457;
- 6bS. R. Chemler, D. Trauner, S. J. Danishefsky, Angew. Chem. 2001, 113, 4676;
10.1002/1521-3757(20011217)113:24<4676::AID-ANGE4676>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4544.10.1002/1521-3773(20011217)40:24<4544::AID-ANIE4544>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 7For reviews of ring-closing metathesis, see:
- 7aA. Fürstner, Angew. Chem. 2000, 112, 3140;
10.1002/1521-3757(20000901)112:17<3140::AID-ANGE3140>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3012;10.1002/1521-3773(20000901)39:17<3012::AID-ANIE3012>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 7bK. C. Nicolaou, P. G. Bulger, D. Sarlah, Angew. Chem. 2005, 117, 4564;
10.1002/ange.200500369 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4490.
- 8O. Mitsunobu, Synthesis 1981, 1.
- 9Y. Yang, J. Janjic, S. A. Kozmin, J. Am. Chem. Soc. 2002, 124, 13670.
- 10J. Inanaga, K. Hirata, H. Saeki, T. Katsuki, M. Yamaguchi, Bull. Chem. Soc. Jpn. 1979, 52, 1989.
- 11For our successful implementation of enol phosphates in palladium-catalyzed synthesis of heterocycles, see:
- 11aM. Sasaki, H. Fuwa, M. Ishikawa, K. Tachibana, Org. Lett. 1999, 1, 1075;
- 11bM. Sasaki, M. Ishikawa, H. Fuwa, K. Tachibana, Tetrahedron 2002, 58, 1889;
- 11cH. Fuwa, N. Kainuma, K. Tachibana, M. Sasaki, J. Am. Chem. Soc. 2002, 124, 14983;
- 11dC. Tsukano, M. Ebine, M. Sasaki, J. Am. Chem. Soc. 2005, 127, 4326;
- 11eH. Fuwa, M. Ebine, A. J. Bourdelais, D. G. Baden, M. Sasaki, J. Am. Chem. Soc. 2006, 128, 16989;
- 11fH. Fuwa, A. Kaneko, Y. Sugimoto, T. Tomita, T. Iwatsubo, M. Sasaki, Heterocycles 2006, 70, 101;
- 11gH. Fuwa, M. Sasaki, Org. Biomol. Chem. 2007, 5, 1849;
- 11hH. Fuwa, M. Sasaki, Chem. Commun. 2007, 2876;
- 11iH. Fuwa, M. Sasaki, Org. Lett. 2007, 9, 3347.
- 12For other examples of the synthesis of endocyclic enol ethers employing RCM, see:
- 12aK. C. Nicolaou, M. H. D. Postema, C. F. Claiborne, J. Am. Chem. Soc. 1996, 118, 1565;
- 12bJ. D. Rainier, S. P. Allwein, J. Org. Chem. 1998, 63, 5310;
- 12cD. Calimente, M. H. D. Postema, J. Org. Chem. 1999, 64, 1770;
- 12dJ. S. Clark, M. C. Kimber, J. Robertson, C. S. P. McErlean, C. Wilson, Angew. Chem. 2005, 117, 6313; Angew. Chem. Int. Ed. 2005, 44, 6157.
- 13H. C. Brown, P. K. Jadhav, J. Am. Chem. Soc. 1981, 105, 2092.
- 14For a review of olefin cross-metathesis, see: S. J. Connon, S. Blechert, Angew. Chem. 2003, 115, 1944;
10.1002/ange.200200556 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1900.
- 15F. Keyling-Bilger, G. Schimitt, A. Beck, B. Luu, Tetrahedron 1996, 52, 14891.
- 16Treatment of lactone 25 with KHMDS or LHMDS resulted in decomposition of the starting material, even in the presence of (PhO)2P(O)Cl, PhNTf2, or Comins reagent (2-[N,N-bis(trifluoromethylsulfonyl)amino]-5-chloropyridine).
- 17E. D. Laganis, B. L. Chenard, Tetrahedron Lett. 1984, 25, 5831.