Journal list menu
Export Citations
Download PDFs
Table of Contents
Heat Transfer Characteristics of CO2 Desorption from N-Methyldiethanolamine Solution in a Microchannel Reactor
- First Published: 02 May 2018
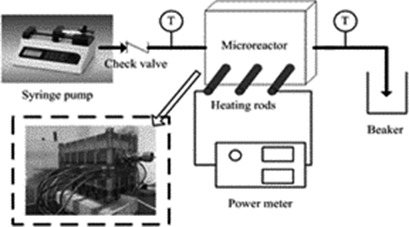
The heat transfer characteristics and energy consumption of CO2 desorption from rich N-methyldiethanolamine solution were evaluated in a highly efficient microchannel reactor. Nucleate boiling was determined as the dominant heat transfer mechanism. The heat transfer coefficients were larger than those reported for CO2 desorption. A correlation was proposed to predict the heat transfer coefficient.
Improving Physical Adsorption of CO2 by Ionic Liquids-Loaded Mesoporous Silica
- First Published: 22 March 2018
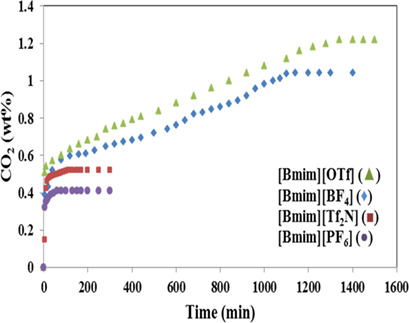
Adsorbents combining a small amount of ionic liquids (ILs) and a large amount of porous solid supports like silica were designed as good alternatives for conventional CO2 capture solvents. Silica-supported ILs were synthesized by impregnation-vaporization. Impregnation of ILs onto the silica support improved the CO2 sorption capacity significantly. CO2-saturated sorbents can be easily recycled.
Increasing Porosity of Molded Calcium-Based Sorbents by Glucose Templating for Cyclic CO2 Capture
- First Published: 02 March 2018
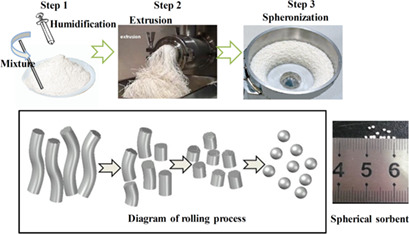
A sorbent modeling technology was applied to produce spherically shaped sorbents with enhanced anti-attrition property for the industrial calcium looping process and glucose was templated to ameliorate the porous structure of calcium-based sorbents. An excellent anti-attrition performance compared to limestone and an improved CO2 capture performance of the templated sorbents could be achieved.
Recent Membrane Developments for CO2 Separation and Capture
- First Published: 05 January 2018
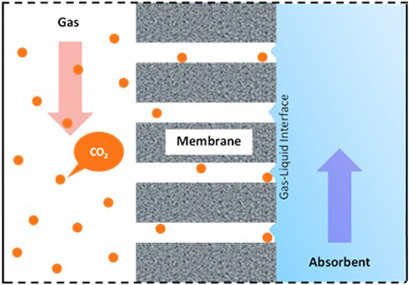
The state-of-the art membrane developments, especially for CO2 separation via the two membrane technologies, namely, membrane gas separation and membrane contactor, are updated. Concepts, challenges, and strategies to tackle the limitations of both membrane technologies are discussed. Future perspectives of these technologies as a potential solution for CO2 removal and utilization are discussed.
Experimental and Numerical Investigation of CO2 Absorption Using Nanofluids in a Hollow-Fiber Membrane Contactor
- First Published: 30 November 2017
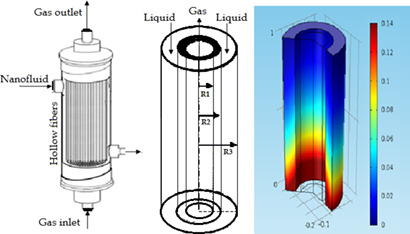
Gas-liquid hollow-fiber membrane contactors are favorable alternatives to conventional absorption techniques for CO2 capture. For removal of CO2 from a N2/CO2 gas mixture, α-Al2O3/water and γ-Al2O3/water nanofluids were applied in a dialysis nanoporous hollow-fiber membrane contactor. The nanofluids enhanced significantly the mass transfer coefficients and absorption rates of CO2.
Power Generation from Coke Oven Gas Using Chemical Looping Combustion: Thermodynamic Simulation
- First Published: 24 November 2017
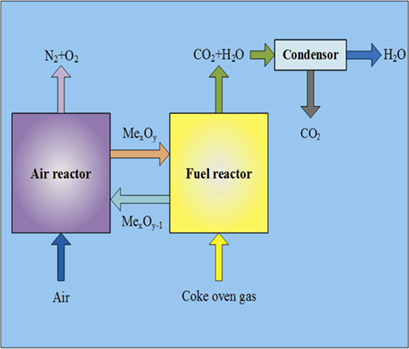
Chemical looping combustion (CLC) is a potentially economic technology allowing for high fuel combustion efficiency with inherent separation of pure CO2. The CLC-combined cycle with coke oven gas as fuel and NiO/NiAl2O4 as an oxygen carrier exhibits high system efficiency with a CO2 capture efficiency of almost 100 % and near-zero carbon emission.
Issues and Current Trends of Hollow-Fiber Mixed-Matrix Membranes for CO2 Separation from N2 and CH4
- First Published: 20 October 2017
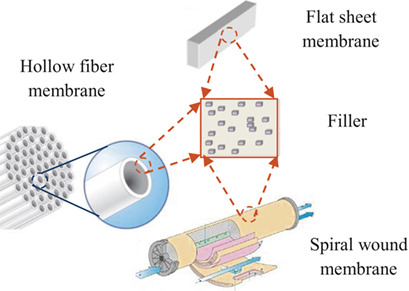
Hollow-fiber mixed-matrix membranes (HFMMMs) possess great potential for CO2 separation. Different inorganic fillers can be incorporated into various types of polymeric materials for fabrication of HFMMMs. Current trends, updated performances, issues on fabrication, and applicable models for prediction of transport properties of HFMMMs in CO2/N2 and CO2/CH4 separation are reviewed in detail.
Chemical Absorption of Carbon Dioxide Using Aqueous Piperidine Derivatives
- First Published: 04 October 2017
The heat of CO2 absorption is a key factor governing the operating cost of the CO2 absorption process when using aqueous amine solutions. Piperidine derivatives were tested in order to identify the effect of the functional groups on CO2 loading and heat of absorption. The introduction of functional groups could be advantageous in CO2 absorption and stripping performance.
Hydrogen Production from Co-Gasification of Coal and Biomass in the Presence of CaO as a Sorbent
- First Published: 26 September 2017
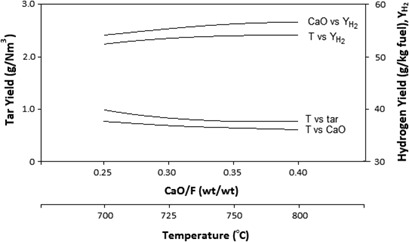
For clean energy production, gasification offers the best combination of investment and value of produced electricity compared to other methods. A parametric study of co-gasification of biomass and coal in a circulation fluidized-bed gasification system was performed using an Aspen Plus simulator. Particular focus was lying on the hydrogen and tar yields obtained by this technology.
Simultaneous Sodium Hydroxide Production by Membrane Electrolysis and Carbon Dioxide Capture
- First Published: 22 September 2017
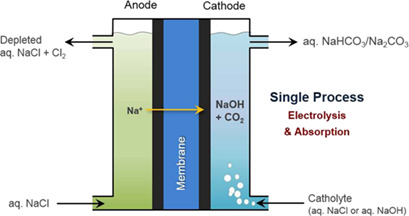
A one-step process for generation of NaOH via electrolysis of NaCl and CO2 absorption simultaneously in an electrochemical device allows reducing the capital cost in the entire process for carbonate mineral production. The simultaneous process was performed in an electrolyzer and produced aqueous carbonate/bicarbonate solutions prior to the mineralization process.
Natural Calcium-Based Residues for Carbon Dioxide Capture in a Bubbling Fluidized-Bed Reactor
- First Published: 21 September 2017
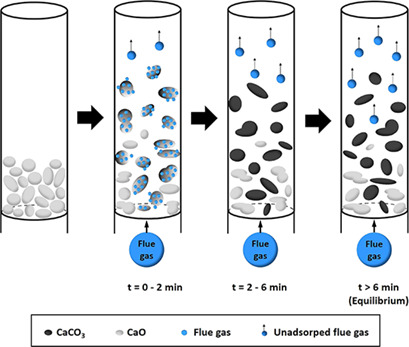
Reducing the amount of industrially emitted CO2 is increasingly important because of global warming. This study investigates calcium oxide as a CO2 adsorbent in fluidized-bed systems. CaO can be generated from the decomposition of calcium carbonate, which is abundant throughout nature. Using such natural calcium-based materials can help reduce agricultural waste and support ecofriendly materials.
Capturing CO2 by Using a Microalgae Culture Recycle Solution
- First Published: 18 September 2017
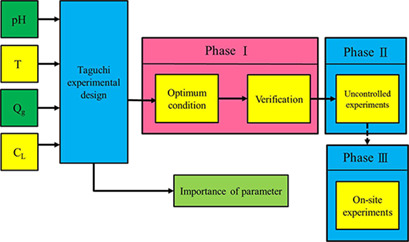
The growth of algae requires CO2 as one of the main nutrients, so there is an opportunity to sequester CO2 by using flue gas emissions from industrial sources as the feed for algae cultivation. A microalgae culture recycle solution was successfully reused to capture CO2 in a continuous bubble-column scrubber under a constant pH environment. Optimum conditions were elaborated.
Absorption of CO2 with Amino Acid-Based Ionic Liquids and Corresponding Amino Acid Precursors
- First Published: 07 August 2017
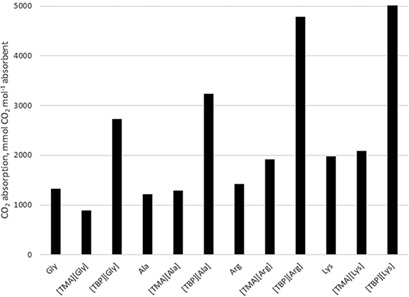
Chemical absorption is an attractive technology for CO2 capture. Among different chemical absorbents suggested for this application, ionic liquids containing amino acids as anions are promising candidates for industrial implementation. A fast and effective method with low energy requirement in terms of microwave-assisted regeneration of the absorbents from the CO2-saturated solution is proposed.
Enhanced CO2 Capture Performance of Limestone by Industrial Waste Sludge
- First Published: 04 August 2017

Commonly applied inert solid supports are considered effective to improve the CO2 capture performance of CaO sorbents, but stem from expensive raw materials. Cheap waste sludge from steel plants is premixed with natural limestone and fed into a calcium looping system. Thereby, both the enhanced CO2 capture performance of limestone and the reuse of the waste industrial sludge could be implemented.
Kinetics of CO2 Adsorption/Desorption of Polyethyleneimine-Mesoporous Silica
- First Published: 07 June 2017
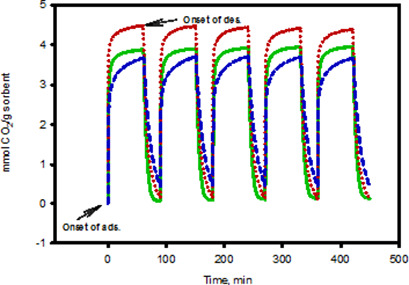
Increasing levels of CO2 emissions from natural gas streams and burned fossil fuels are a serious environmental concern. Therefore, removal of CO2 is necessary. A kinetic investigation of CO2 adsorption/desorption has been carried out on sorbents with and without surfactants. Unlike most of the previous kinetic treatments reported, the present model aims to specifically distinguish between surface and bulk adsorption processes.









