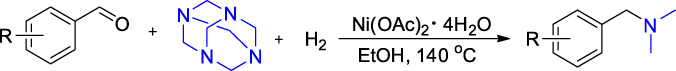Journal list menu
Export Citations
Download PDFs
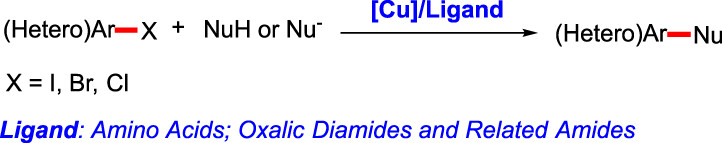
Cover Picture
Cover Picture
- Page: 793
- First Published: 15 July 2020

The cover picture shows the copper-catalyzed Ullmann-Ma reaction of aryl halides with nucleophiles by the assistance of two-generation ligands, amino acids and oxalic diamides or related amides. Ullmann-Ma reaction is one of the most important transformations for the construction of aryl carbon-carbon and carbon-heteroatom bonds in organic chemistry. For the details about the history, development, scope and applications of Ullmann-Ma reaction, please see the article by Cai et al. on page 879–893.
Inside Cover
Inside Cover
- Page: 794
- First Published: 15 July 2020

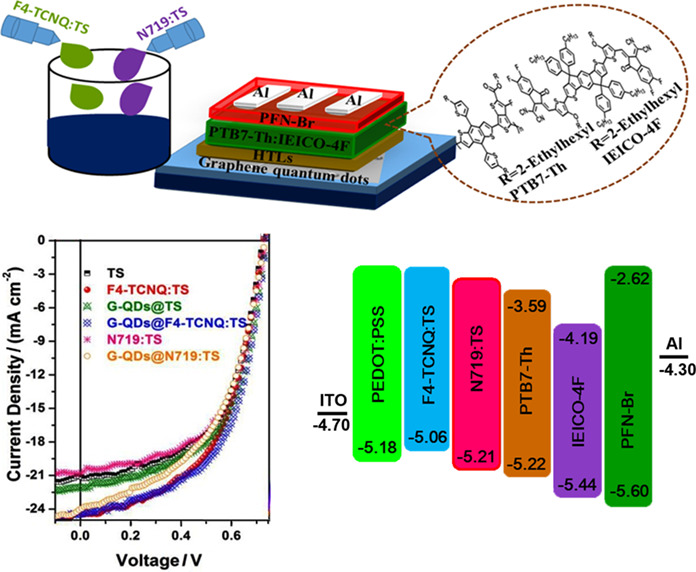
Interfacial engineering is expected to be a feasible strategy to improve the charge transport properties of the hole transport layer, which is of crucial importance to boost the device performance of organic solar cells. In this study, the doping of F4-TCNQ and N719 dye in the hole transport layer with and without integrating a graphene quantum-dots layer has been explored in non-fullerene acceptor-based organic solar cells. More details are discussed in the article by Feng et al. on page 817–822.
Contents
Concise Reports
Radical Heteroarylalkylation of Alkenes via Three-Component Docking-Migration Thioetherification Cascade
- Pages: 803-806
- First Published: 14 March 2020
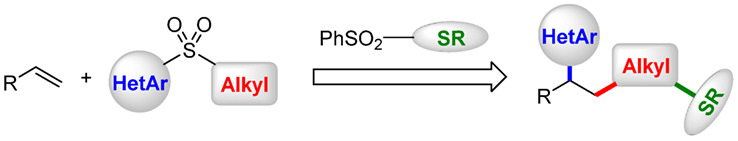
A novel, rational-designed approach to access various heteroaryl-substituted alkyl thioethers was developed via docking-migration cascade process. By utilizing three components involving alkene, dual-function reagent, and thioetherificating reagent, radical heteroarylalkylation of alkenes followed by thiolation of the alkyl radical intermediates proceeded smoothly, manifesting well compatibility of substrates and cascade transformations. Furthermore, this protocol also features mild conditions, broad substrate scope, and wide product diversity.
Sequential Ir-Catalyzed Allylation/2-aza-Cope Rearrangement Strategy for the Construction of Chiral Homoallylic Amines†
- Pages: 807-811
- First Published: 14 March 2020
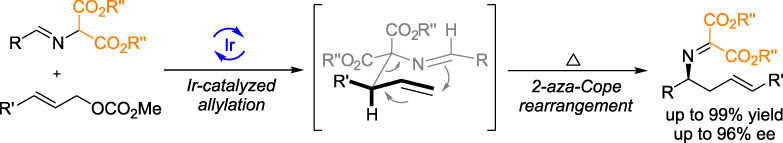
Sequential Ir-catalyzed asymmetric allylation/2-aza-Cope rearrangement of arylidene amino-malonates with allylic carbonates was successfully developed, and a variety of enantioenriched homoallylic amine derivatives were obtained in high yields with good chirality transfer and excellent E/Z-geometry control (up to 99% yield, 96% ee). Compared with previous dual catalytic system established for this transformation, the current mono metal catalytic system provides a simpler and more practical protocol employing the readily available starting materials.
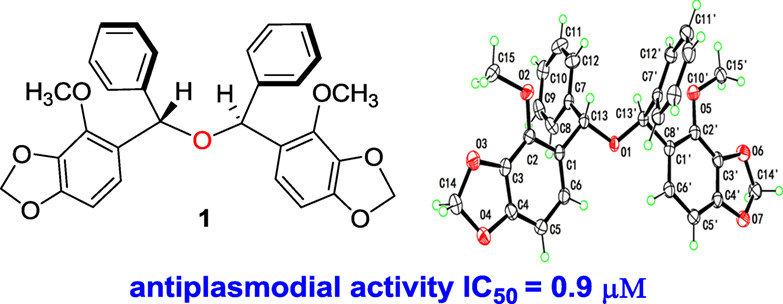
Structurally Interesting Diarymethane Derivatives from Securidaca inappendiculata
- Pages: 812-816
- First Published: 13 March 2020
Modification of Hole Transport Layers for Fabricating High Performance Non-fullerene Polymer Solar Cells
- Pages: 817-822
- First Published: 29 March 2020
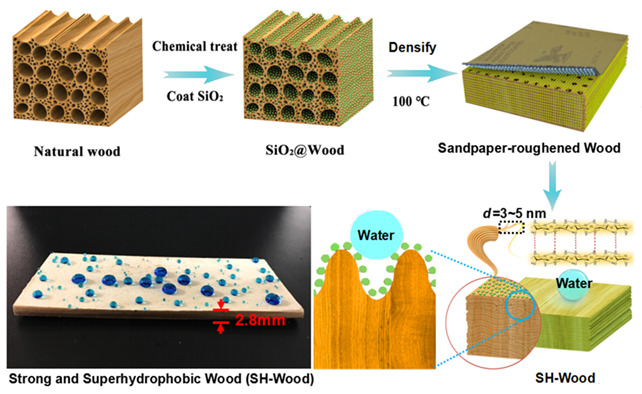
Strong and Superhydrophobic Wood with Aligned Cellulose Nanofibers as a Waterproof Structural Material†
- Pages: 823-829
- First Published: 11 March 2020
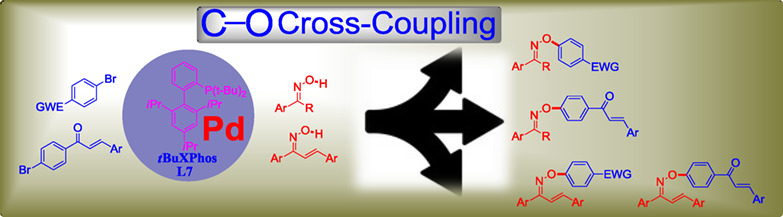
An Easy Access to Oxime Ethers by Pd-Catalyzed C—O Cross-Coupling of Activated Aryl Bromides with Ketoximes and Chalcone Oximes
- Pages: 830-836
- First Published: 17 March 2020
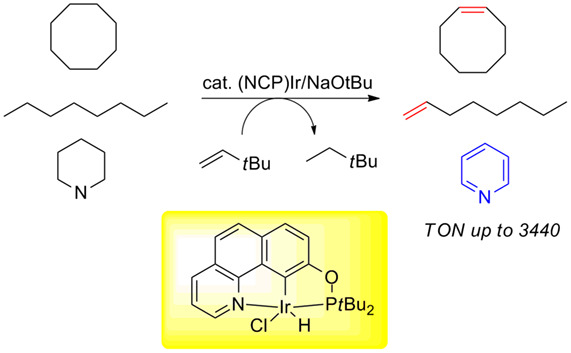
NCP-Type Pincer Iridium Complexes Catalyzed Transfer-Dehydrogenation of Alkanes and Heterocycles†
- Pages: 837-841
- First Published: 18 March 2020
A Novel Route to Synthesize N,N-Dimethyl Arylmethylamines from Aryl Aldehydes, Hexamethylenetetramine and Hydrogen†
- Pages: 842-846
- First Published: 18 March 2020
Synthesis and Properties of CF3(OCF3)CH-Substituted Arenes and Alkenes†
- Pages: 847-854
- First Published: 14 March 2020
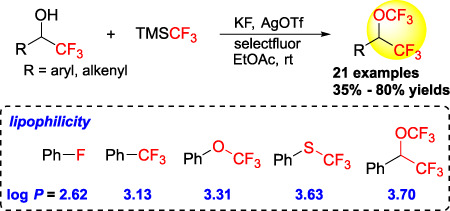
A series of novel CF3(OCF3)CH-substituted arenes and alkenes were prepared through the silver-mediated oxidative trifluoromethylation of α-trifluoromethyl alcohols. Evaluation of the lipophilicities indicated that this CH(OCF3)CF3 motif is more lipophilic than the common fluorine-containing groups such as CF3, OCF3, and SCF3.
Recent Advances
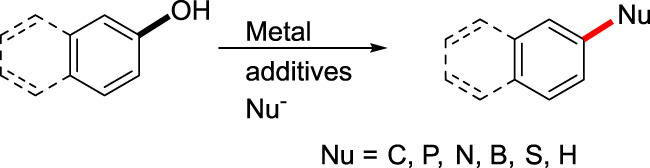
Direct Transformation of Arenols Based on C—O Activation
- Pages: 855-863
- First Published: 18 March 2020
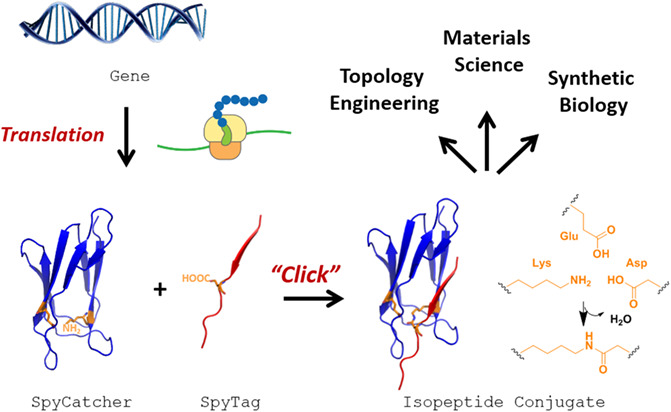
Encrypting Chemical Reactivity in Protein Sequences toward Information-Coded Reactions†
- Pages: 864-878
- First Published: 05 April 2020
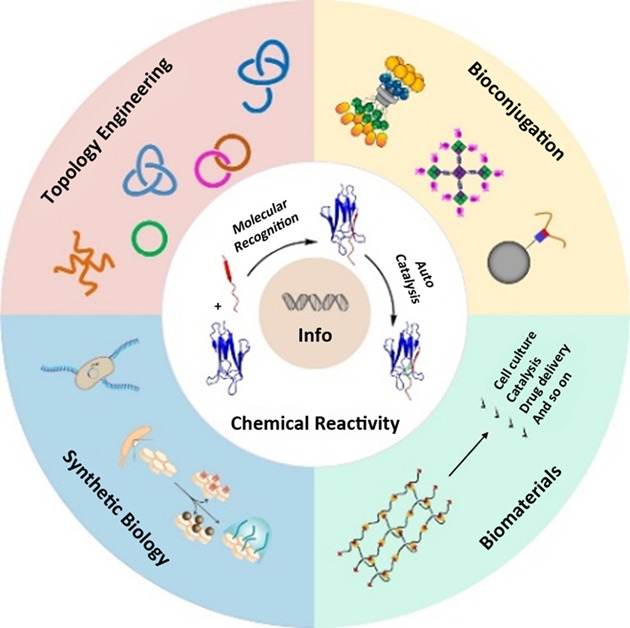
We summarize the recent development of genetically encoded peptide-protein coupling reactions and their diverse applications. It represents a novel way to tune chemical reactivity by the information encoded in the protein sequence, which is read out by protein folding and molecular recognition and translated into chemical bonding. The principles may be extended to enable new modes of supramolecular interactions that are coupled with covalent bonding.
Cornerstones in Chemistry
Ullmann-Ma Reaction: Development, Scope and Applications in Organic Synthesis†
- Pages: 879-893
- First Published: 27 March 2020
Emerging Topic
Inside Story
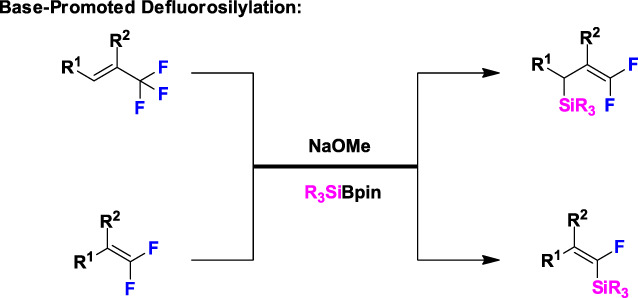
Catalyst-Free Defluorosilylation for Synthesis of Silylated gem-Difluoroalkenes and Monofluoroalkenes
- Pages: 897-898
- First Published: 14 April 2020