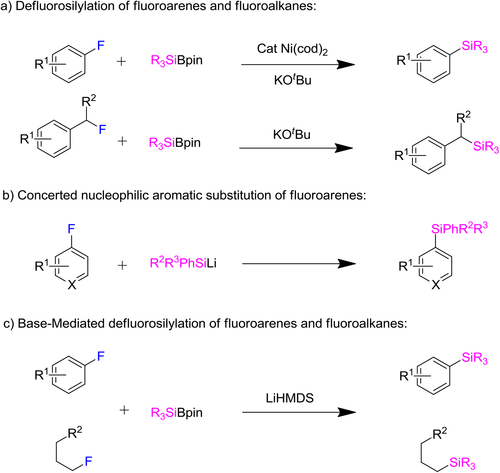
Catalyst-Free Defluorosilylation for Synthesis of Silylated gem-Difluoroalkenes and Monofluoroalkenes
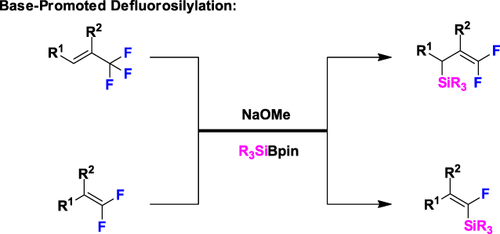
Graphical Abstract
Fluorine containing compounds have an extensive range of application in pharmaceutical and agrochemical fields, in which the C—F bond takes a decisive role.[1] Due to this fact, it is great motivation for chemists to develop expedient synthetic methods for the synthesis of structurally expanded fluorinated compounds. Indeed, cleavage of unactivated C—F bonds is a challenge to make new molecular skeletons. A series of defluorinative functionalization of unactivated fluoroarenes and fluoroalkanes have been reported. For example, Shibata group reported Ni-catalyzed defluorosilylation of fluoroarenes and subsequently alkyl fluorides were successfully shown to convert into alkyl silanes without Ni catalyst (Scheme 1a).[2] After a short while, notable contributions from Studer and Würthwein group have presented concerted nucleophilic substitution of various fluoroarenes in the presence of silyl lithium reagents (Scheme 1b).[3] At the same time, Martin and coworkers productively realized base-promoted defluorosilylation of fluoroarenes and fluoroalkanes by C—F bond cleavage in the absence of transition metals or specialized ligands (Scheme 1c).[4] Although synthesis of aryl and alkyl silanes through C—F bond cleavage made great achievement, the transition-metal-free defluorosilylation of fluoroalkenes to build silylated fluoroalkene is still challenging.
In 2018, our group reported copper and NHC catalyzed asymmetric synthesis of gem-difluoroallylboronates from 1-(trifluoromethyl)alkenes via defluoroborylation strategy in the first time. Fruitfully, this method provided a series of highly stereoselective gem-difluoroallylboronates in good to excellent yields (Scheme 2a).[5] Subsequently, we have showcased an copper-catalytic
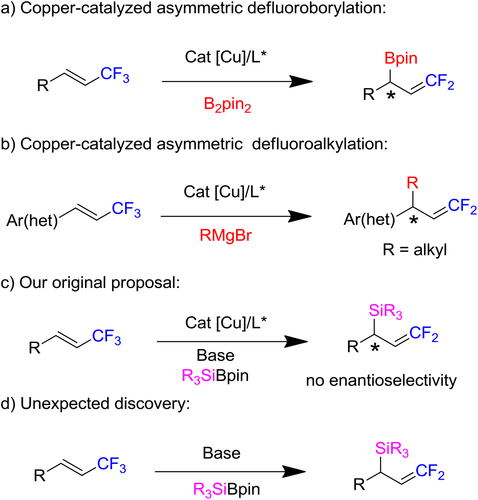
system for defluoroalkylation to generate various gem-difluoroalkenes from arylboronate using activated alkyl Grignard reagents (Scheme 2b).[6] Inspired by the shortcoming of defluorosilylation and our two works,[7] we deliberated to synthesis of stereoselective silylated fluoroalkenes from 1-(trifluoromethyl)alkenes through defluorosilylation process. However, with our continuous effort, we detected the desired silylated fluoroalkenes with no stereoselectivity (Scheme 2c). Surprisingly, in the absence of copper catalyst, we could observe the same product in good yield. This method is rather efficient, scalable and simple for the synthesis of silylated fluoroalkenes, in which the silyl group is readily elaborated to provide various functionalized fluoroalkene derivatives. Based on this discovery, herein we report an unexpected catalyst-free system to activate the C—F bonds of fluoroalkenes via a defluorosilylation process to produce a series of silylated fluoroalkenes in good to excellent yield (Scheme 2d).[8]
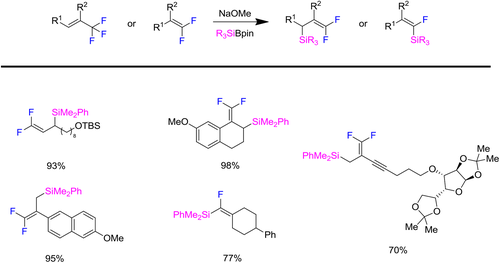
This reaction shows excellent substrate compatibility. Neither 1-(trifluoromethyl)alkenes nor (3,3,3-trifluoroprop-1-en-2-yl)alkenes can be transformed into their corresponding gem-difluoroalkenes (Scheme 3). Furthermore, internal trifluoromethyl alkenes colud also convert into desired products in good yields. Besides, sterically hindered tetrasubstituted gem-difluoroalkenes can proceed efficiently to build silylated monofluoroalkenes. Most importantly, this method can be applied to late-stage modification of biologically relevant complex molecules.
In conclusion, we have established an expedient synthetic strategy, ie., catalyst-free defluorosilylation for the synthesis of silylated gem-difluoroalkenes and monofluoroalkenes. However, we will stay true to the mission to realize chiral silylated fluoroalkenes via similar protocol. Despite the enormous challenges, relevant research is under way in our laboratory. It is conceivable that chiral silylated gem-difluoroalkenes will be realized in the near future.