Direct Transformation of Arenols Based on C—O Activation
Feng Liu
School of Perfume and Aroma Technology, Shanghai Institute of Technology, 100 Haiquan Rd, Shanghai, 201418 China
Department of Chemistry, Fudan University, 2005 Honghu Rd, Shanghai, 200438 China
Search for more papers by this authorHao-jun Jiang
School of Perfume and Aroma Technology, Shanghai Institute of Technology, 100 Haiquan Rd, Shanghai, 201418 China
Search for more papers by this authorYi Zhou
School of Perfume and Aroma Technology, Shanghai Institute of Technology, 100 Haiquan Rd, Shanghai, 201418 China
Search for more papers by this authorCorresponding Author
Zhang-jie Shi
Department of Chemistry, Fudan University, 2005 Honghu Rd, Shanghai, 200438 China
E-mail: [email protected]Search for more papers by this authorFeng Liu
School of Perfume and Aroma Technology, Shanghai Institute of Technology, 100 Haiquan Rd, Shanghai, 201418 China
Department of Chemistry, Fudan University, 2005 Honghu Rd, Shanghai, 200438 China
Search for more papers by this authorHao-jun Jiang
School of Perfume and Aroma Technology, Shanghai Institute of Technology, 100 Haiquan Rd, Shanghai, 201418 China
Search for more papers by this authorYi Zhou
School of Perfume and Aroma Technology, Shanghai Institute of Technology, 100 Haiquan Rd, Shanghai, 201418 China
Search for more papers by this authorCorresponding Author
Zhang-jie Shi
Department of Chemistry, Fudan University, 2005 Honghu Rd, Shanghai, 200438 China
E-mail: [email protected]Search for more papers by this author†Dedicated to the 30th Anniversary of State Key Laboratory of Organometallic Chemistry.
Abstract
In the view of substrate availability, atomic efficiency and cost, directly using arenols as coupling partners in cross-coupling, would be one of the most attractive goals. Up to date, many efforts have been made to activate the C—O bond of phenols with different strategies, for example, through in-situ formed intermediates, through a catalytic reductive dearomatization-condensation-rearomatization sequence or catalytic deoxygenation. In this review, we summarized recent advances in cross-couplings of arenols as the electrophiles via C—O activation.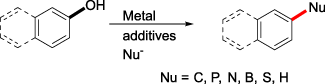
References
- 1(a) Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483;
(b) Negishi, E. Magical Power of Transition Metals: Past, Present, and Future (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6738–6764;
(c) Cross-Coupling Reactions. A Practical Guide, Ed.: Miyaura, N., Springer-Verlag, Berlin, 2002;
(d) Metal-Catalyzed Cross-Coupling Reactions, 2nd ed., Eds: de Mejeire, A.; Diedrich, F., Wiley-VCH, Weinheim, Germany, 2004;
(e) Tsuji, J. Palladium Reagents and Catalysts: New Perspectives for the 21st Century, John Wiley & Sons, Ltd., New York, 2004;
10.1002/0470021209 Google Scholar(f) Nicolaou, K. C.; Bulger, P. H.; Sarlah, D. Palladium-Catalyzed Cross-Coupling Reactions in Total Synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489; (g) Littke, A. F.; Fu, G. C. Palladium-Catalyzed Coupling Reactions of Aryl Chlorides. Angew. Chem. Int. Ed. 2002, 41, 4176–4211.10.1002/1521-3773(20021115)41:22<4176::AID-ANIE4176>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 2(a) Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl- Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1470; (b) Stanforth, S. P. Catalytic Cross-Coupling Reactions in Biaryl Synthesis. Tetrahedron 1998, 54, 263–303; (c) Brown, B. R. The Organic Chemistry of Aliphatic Nitrogen Compounds, Cambridge University, Cambridge, 2004; (d) McElroy, W. T.; DeShong, P. Synthesis of the CD-ring of the Anticancer Agent Streptonigrin: Studies of Aryl-Aryl Coupling Methodologies. Application of a Catalytic Palladium Biaryl Synthesis Reaction, via C-H Functionalization, to the Total Synthesis of Amaryllidaceae Alkaloids. Tetrahedron 2006, 62, 6945–6954; (e) Torres, J. C.; Pinto, A. C.; Garden, S. J. Application of a Catalytic Palladium Biaryl Synthesis Reaction, via C-H Functionalization, to the Total Synthesis of Amaryllidaceae Alkaloids. Tetrahedron 2004, 60, 9889–9900; (f) Lawerence, S. A. Amines: Synthesis, Properties and Applications , Cambridge University, Cambridge, 2004; (g) Hajduk, P. J.; Bures, M.; Praestgaard, J.; Fesik, S. W. J. Privileged Molecules for Protein Binding Identified from NMR-Based Screening. J. Med. Chem. 2000, 43, 3443–3447
- 3(a) Metal-Catalyzed Cross-Coupling Reactions, Eds.: Diederich, F.; Stang, P. J., Wiley-VCH, Weinheim, Germany, 1998; (b) Cross-Coupling Reactions, A Practical Guide, Ed.: Miyaura, N., SpringerVerlag, Berlin, 2002; (c) Metal-Catalyzed Cross-Coupling Reactions, Eds.: de Meijere, A.; Diederich, F., Wiley-VCH, Weinheim, Germany, 2004; (d) Topics in Current Chemistry, Vol. 219, Ed. Miyaura, N., Springer Verlag, New York, 2002; (e) Corbet, J. P.; Mignani, G. Selected Patented Cross-Coupling Reaction Technologies. Chem. Rev. 2006, 106, 2651–2710; (f) Negishi, E. Transition Metal- Catalyzed Organometallic Reactions that Have Revolutionized Organic Synthesis. Bull. Chem. Soc. Jpn. 2007, 80, 233–257; (g) Negishi, E. Palladium- or Nickel-Catalyzed Cross Coupling. A New Selective Method for Carbon-Carbon Bond Formation. Acc. Chem. Res. 1982, 15, 340–348; (h) Zhang, Y. F.; Shi, Z. J. Upgrading Cross-Coupling Reactions for Biaryl Syntheses. Acc. Chem. Res. 2019, 52, 161–169.
- 4(a) The Chemistry of Phenols, Ed.: Rappoport, Z., John Wiley & Sons Ltd., Chichester, UK, 2003; (b) Tyman, J. H. P. Synthetic and Natural Phenols, Elsevier, Amsterdam, 1996.
- 5For selected reviews on C-O electrophiles, see: (a) Mesganaw, T.; Garg, N. K. Ni-and Fe-Catalyzed Cross-Coupling Reactions of Phenol Derivatives. Org. Process Res. Dev. 2013, 17, 29–39; (b) Yamaguchi, J.; Muto, K.; Itami, K. Recent Progress in Nickel-Catalyzed Biaryl Coupling. Eur. J. Org. Chem. 2013, 19–30; (c) Correa, A.; Cornella, J.; Martin, R. Nickel-Catalyzed Decarbonylative C-H Coupling Reactions: A Strategy for Preparing Bis(heteroaryl) Backbones. Angew. Chem. Int. Ed. 2013, 52, 1878–1880; (d) Rosen, B. M.; Quasdorf, K. W.; Wilson, D. A.; Zhang, N.; Resmerita, A. M.; Garg, N. K.; Percec, V. Nickel-Catalyzed Cross-Couplings Involving Carbon-Oxygen Bonds. Chem. Rev. 2011, 111, 1346–1416; (e) Li, B. J.; Yu, D. G.; Sun, C. L.; Shi, Z. J. Activation of “Inert” Alkenyl/Aryl C-O Bond and Its Application in Cross-Coupling Reactions. Chem. Eur. J. 2011, 17, 1728–1759; (f) Yu, D. G.; Li, B. J.; Shi, Z. J. Exploration of New C-O Electrophiles in Cross-Coupling Reactions. Acc. Chem. Res. 2010, 43, 1486–1495; (g) Cornella, J.; Zarate, C.; Martin, R. Metal-Catalyzed Activation of Ethers via C-O Bond Cleavage: A New Strategy for Molecular Diversity. Chem. Soc. Rev. 2014, 43, 8081–8097; (h) Tobisu, M.; Chatani, N. Cross-Couplings Using Aryl Ethers via C-O Bond Activation Enabled by Nickel Catalysts. Acc. Chem. Res. 2015, 48, 1717–1726; (i) Tasker, S. Z.; Standley, E. A.; Jamison, T. F. Recent Advances in Homogeneous Nickel Catalysis. Nature 2014, 509, 299–309; (j) Zeng, H. Y.; Qiu, Z. H.; Dominguez-Huerta, A.; Hearne, Z.; Chen, Z. W.; Li, C. J. An Adventure in Sustainable Cross-Coupling of Phenols and Derivatives via Carbon-Oxygen Bond Cleavage. ACS Catal. 2017, 7, 510–519; (k) Zaratea, C.; Gemmerena, M. V.; Somerville, R. J.; Martin, R. in Advances in Organometallic Chemistry, Volume 66, Chapter 4, Ed.: Perez, P. J., Elsevier, Amsterdam, 2016.
- 6(a) Hayashi, T.; Katsuro, Y.; Okamoto, Y.; Kumada, M. Nickel-Catalyzed Cross-Coupling of Aryl Phosphates with Grignard and Organoaluminium Reagents. Synthesis of Alkyl-, Alkenyl-, and Arylbenzenes from Phenols. Tetrahedron Lett. 1981, 22, 4449–4452; (b) Yoshikai, N.; Matsuda, H.; Nakamura, E. J. Hydroxyphosphine Ligand for Nickel-Catalyzed Cross-Coupling through Nickel/Magnesium Bimetallic Cooperation. J. Am. Chem. Soc. 2009, 131, 9590–9599; (c) Tanaka, M.; Chiba, K. I.; Okita, M.; Kaneko, T.; Tagami, K.; Hibi, S.; Okamoto, Y.; Shirota, H.; Goto, M.; Obaishi, H.; Sakurai, H.; Machida, Y.; Yamatsu, I. J. A Novel Orally Active Inhibitor of IL-1 Generation: Synthesis and Structure-Activity Relationships of 3-(4-Hydroxy-1- Naphthalenyl)-2-Propenoic Acid Derivatives. J. Med. Chem. 1992, 35, 4665–4675; (d) Iwashima, M.; Nagaoka, H.; Kobayashi, K.; Yamada, Y. Total Synthesis of Marine Diterpene Fuscol. Tetrahedron Lett. 1992, 33, 81–82; (e) Chen, H.; Huang, Z.; Hu, X.; Tang, G.; Xu, P.; Zhao, Y.; Cheng, C. H. Nickel-Catalyzed Cross-Coupling of Aryl Phosphates with Arylboronic Acids. J. Org. Chem. 2011, 76, 2338–2344; (f) Zhao, Y. L.; Li, Y.; Gao, L. X.; Han, F. S. Aryl Phosphoramides: Useful Electrophiles for Suzuki-Miyaura Coupling Catalyzed by a NiCl2/dppp System (dppp=1,3-Bis(diphenylphosphino)propane). Chem. Eur. J. 2010, 16, 4991–4994; (g) Huang, J. H.; Yang, L. M. Nickel-Catalyzed Amination of Aryl Phosphates through Cleaving Aryl C-O Bonds. Org. Lett. 2011, 13, 3750–3753; (h) De Carolis, M.; Protti, S.; Fagnoni, M.; Albini, A. Metal-Free Cross-Coupling Reactions of Aryl Sulfonates and Phosphates through Photoheterolysis of Aryl-Oxygen Bonds. Angew. Chem. Int. Ed. 2005, 44, 1232–1236; (i) Dichiarante, V.; Fagnoni, M.; Albini, A. Metal-Free Synthesis of Sterically Crowded Biphenyls by Direct Ar-H Substitution in Alkyl Benzenes. Angew. Chem. Int. Ed. 2007, 46, 6495–6498
- 7(a) Sengupta, S.; Leite, M.; Raslan, D. S.; Quesnelle, C.; Snieckus, V. J. Nickel (0)-Catalyzed Cross Coupling of Aryl O-Carbamates and Aryl Triflates with Grignard Reagents. Directed Ortho Metalation-Aligned Synthetic Methods for Polysubstituted Aromatics via a 1,2-Dipole Equivalent. J. Org. Chem. 1992, 57, 4066–4069; (b) Dallaire, C.; Kolber, I.; Gingras, M. Nickel-Catalyzed Coupling of Aryl O-Carbamates with Grignard Reagents: 2,7-Dimethylnaphthalene. Org. Synth. 2002, 78, 42; (c) Yoshikai, N.; Matsuda, H.; Nakamura, E. Hydroxyphosphine Ligand for Nickel-Catalyzed Cross-Coupling through Nickel/Magnesium Bimetallic Cooperation. J. Am. Chem. Soc. 2009, 131, 9590–9599; (d) Takise, R.; Itami, K.; Yamaguchi, J. Cyanation of Phenol Derivatives with Aminoacetonitriles by Nickel Catalysis. Org. Lett. 2016, 18, 4428–4431; (e) Huang, K.; Yu, D. G.; Zheng, S. F.; Wu, Z. H.; Shi, Z. J. Borylation of Aryl and Alkenyl Carbamates through Ni-Catalyzed C-O Activation. Chem. Eur. J. 2011, 17, 786–791; (f) Antoft-Finch, A.; Blackburn, T.; Snieckus, V. N,N-Diethyl O-Carbamate: Directed Metalation Group and Orthogonal Suzuki-Miyaura Cross- Coupling Partner. J. Am. Chem. Soc. 2009, 131, 17750–17752; (g) Shi, W. J.; Zhao, H. W.; Wang, Y.; Cao, Z. C.; Zhang, L. S.; Yu, D. G.; Shi, Z. J. Nickel- or Iron-Catalyzed Cross-Coupling of Aryl Carbamates with Arylsilanes. Adv. Synth. Catal. 2016, 358, 2410–2416; (h) Wang, Y.; Wu, S. B.; Shi, W. J.; Shi, Z. J. C-O/C-H Coupling of Polyfluoroarenes with Aryl Carbamates by Cooperative Ni/Cu Catalysis. Org. Lett. 2016, 18, 2548–2551; (i) Song, W.; Ackermann, L. Cobalt-Catalyzed Direct Arylation and Benzylation by C-H/C-O Cleavage with Sulfamates, Carbamates, and Phosphates. Angew. Chem. Int. Ed. 2012, 51, 8251–8254; (j) Quasdorf, K. W.; Riener, M.; Petrova, K. V.; Garg, N. K. Suzuki-Miyaura Coupling of Aryl Carbamates, Carbonates, and Sulfamates. J. Am. Chem. Soc. 2009, 131, 17748–17749; (k) Antoft-Finch, T.; Blackburn, V.; Snieckus, J. N,N-Diethyl O-Carbamate: Directed Metalation Group and Orthogonal Suzuki-Miyaura Cross-Coupling Partner. J. Am. Chem. Soc. 2009, 131, 17750–17752; (l) Xu, L.; Li, B. J.; Wu, Z. H.; Lu, X. Y.; Guan, B. T.; Wang, B. Q.; Zhao, K. Q.; Shi, Z. J. Nickel- Catalyzed Efficient and Practical Suzuki-Miyaura Coupling of Alkenyl and Aryl Carbamates with Aryl Boroxines. Org. Lett. 2010, 12, 884–887; (m) Molander, G. A.; Beaumard, F. Nickel-Catalyzed C-O Activation of Phenol Derivatives with Potassium Heteroaryltrifluoroborates. Org. Lett. 2010, 12, 4022–4025; (n) Kuwano, R.; Shimizu, R. An Improvement of Nickel Catalyst for Cross-coupling Reaction of Arylboronic Acids with Aryl Carbonates by Using a Ferrocenyl Bisphosphine Ligand. Chem. Lett, 2011, 40, 913–915.
- 8 Silberstein, A. L.; Ramgren, S. D.; Garg, N. K. Iron-Catalyzed Alkylations of Aryl Sulfamates and Carbamates. Org. Lett. 2012, 14, 3796–3799.
- 9(a) Quadorf, K. W.; Tian, X.; Garg, N. K. Cross-Coupling Reactions of Aryl Pivalates with Boronic Acids. J. Am. Chem. Soc. 2008, 130, 14422–14423; (b) Guan, B. T.; Wang, Y.; Li, B. J.; Yu, D. G.; Shi, Z. J. Biaryl Construction via Ni-Catalyzed C-O Activation of Phenolic Carboxylates. J. Am. Chem.Soc. 2008, 130, 14468–14470; (c) Li, B. J.; Li, Y. Z.; Lu, X. Y.; Liu, J.; Guan, B. T.; Shi, Z. J. Cross-Coupling of Aryl/ Alkenyl Pivalates with Organozinc Reagents through Nickel-Catalyzed C-O Bond Activation under Mild Reaction Conditions. Angew. Chem. Int. Ed. 2008, 47, 10124–10127; (d) Ehle, A. R.; Zhou, Q.; Watson, M. P. Nickel(0)-Catalyzed Heck Cross-Coupling via Activation of Aryl C-O Piv Bonds. Org. Lett. 2012, 14, 1202–1205; (e) Takise, R.; Muto, K.; Yamaguchi, J.; Itami, K. Nickel-Catalyzed α-Arylation of Ketones with Phenol Derivatives. Angew. Chem. Int. Ed. 2014, 53, 6791–6794; (f) Cornella, J.; Jackson, E. P.; Martin, R. Nickel-Catalyzed Enantioselective C-C Bond Formation through Csp2-O Cleavage in Aryl Esters. Angew. Chem. Int. Ed. 2015, 54, 4075–4078; (g) Koch, E.; Takise, R.; Studer, A.; Yamaguchi, J.; Itami, K. Ni-Catalyzed α-Arylation of Esters and Amides with Phenol Derivatives. Chem. Commun. 2015, 51, 855–857; (h) Zarate, C.; Martin, R. A Mild Ni/Cu-Catalyzed Silylation via C-O Cleavage. J. Am. Chem. Soc. 2014, 136, 2236–2239; (i) Correa, A.; Leon, T.; Martin, R. Ni-Catalyzed Carboxylation of C(sp2)- and C(sp3)-O Bonds with CO2. J. Am. Chem. Soc. 2014, 136, 1062–1069; (j) Yang, J.; Chen, T.; Han, L. B. C-P Bond-Forming Reactions via C-O/P-H Cross-Coupling Catalyzed by Nickel. J. Am. Chem. Soc. 2015, 137, 1782–1785; (k) Yang, J.; Xiao, J.; Chen, T.; Han, L. B. Nickel-Catalyzed Phosphorylation of Phenol Derivatives via C-O/P-H Cross-Coupling. J. Org. Chem. 2016, 81, 3911–3916; (l) Guo, L.; Hsiao, C. C.; Yue, H.; Liu, X.; Rueping, M. Nickel-Catalyzed Csp2-Csp3 Cross-Coupling via C-O Bond Activation. ACS Catal. 2016, 6, 4438–4442; (m) Wang, J.; Ferguson, D. M.; Kalyani, D. Nickel-Catalyzed Intramolecular C-H Arylation Using Aryl Pivalates as Electrophiles. Tetrahedron. 2013, 69, 5780–5790; (n) Shimasaki, T.; Tobisu, M.; Chatani, N. Nickel-Catalyzed Amination of Aryl Pivalates by the Cleavage of Aryl C-O Bonds. Angew. Chem. Int. Ed. 2010, 49, 2929–2932; (o) Muto, K.; Yamaguchi, J.; Itami, K. Nickel-Catalyzed C-H/C-O Coupling of Azoles with Phenol Derivatives. J. Am. Chem. Soc. 2012, 134, 169–172; (p) Muto, K.; Hatakeyama, T.; Yamaguchi, J.; Itami, K. C-H Arylation and Alkenylation of Imidazoles by Nickel Catalysis: Solvent-Accelerated Imidazole C-H Activation. Chem. Sci. 2015, 6, 6792–6798; (q) Kinuta, H.; Hasegawa, J.; Tobisu, M.; Chatani, N. Rhodium-catalyzed Borylation of Aryl and Alkenyl Pivalates through the Cleavage of Carbon-Oxygen Bonds. Chem. Lett. 2015, 44, 366–368; (r) Ma, H. P.; Bai, C. L. W.; Bao, Y. S. Nano-Gold Catalyzed Transesterification of (Hetero)aryl Esters with Alkyl Halides via C-O Activation. Chin. J. Chem. 2019, 39, 734–746.
- 10(a) Wenkert, E.; Michelotti, E. L.; Swindell, C. S. Nickel-Induced Conversion of Carbon-Oxygen into Carbon-Carbon Bonds. One-step Transformations of Enol Ethers into Olefins and Aryl Ethers into Biaryls. J. Am. Chem. Soc. 1979, 101, 2246–2247; (b) Dankwardt, J. W. Nickel-Catalyzed Cross-Coupling of Aryl Grignard Reagents with Aromatic Alkyl Ethers: An Efficient Synthesis of Unsymmetrical Biaryls. Angew. Chem. Int. Ed. 2004, 43, 2428–2432; (c) Guan, B. T.; Xiang, S. K.; Wu, T.; Sun, Z. P.; Wang, B. Q. ; Zhao, K. Q.; Shi, Z. J. Methylation of arenes via Ni-Catalyzed Aryl C-O/F Activation. Chem. Commun. 2008, 1437–1439; (d) Tobisu, M.; Shimasaki, T.; Chatani, N. Nickel- Catalyzed Cross-Coupling of Aryl Methyl Ethers with Aryl Boronic Esters. Angew. Chem. Int. Ed. 2008, 47, 4866–4869; (e) Tobisu, M.; Yasutome, A.; Kinuta, H.; Nakamura, K.; Chatani, N. 1,3-Dicyclohexylimidazol-2-ylidene as a Superior Ligand for the Nickel-Catalyzed Cross-Couplings of Aryl and Benzyl Methyl Ethers with Organoboron Reagents. Org. Lett. 2014, 16, 5572–5575; (f) Tobisu, M.; Takahira, T.; Chatani, N. Nickel-Catalyzed Cross-Coupling of Anisoles with Alkyl Grignard Reagents via C-O Bond Cleavage. Org. Lett. 2015, 17, 4352–4355; (g) Tobisu, M.; Takahira, T.; Morioka, T.; Chatani, N. Nickel- Catalyzed Alkylative Cross-Coupling of Anisoles with Grignard Reagents via C-O Bond Activation. J. Am. Chem. Soc. 2016, 138, 6711–6714; (h) Tobisu, M.; Shimasaki, T.; Chatani, N. Ni0-Catalyzed Direct Amination of Anisoles Involving the Cleavage of Carbon-Oxygen Bonds. Chem. Lett. 2009, 38, 710–711; (i) Tobisu, M.; Yasutome, A.; Yamakawa, K.; Shimasaki, T.; Chatani, N. Ni(0)/NHC-Catalyzed Amination of N-Heteroaryl Methyl Ethers through the Cleavage of Carbon–Oxygen Bonds. Tetrahedron 2012, 68, 5157–5161; (j) Wang, C.; Ozaki, T.; Takita, R.; Uchiyama, M. Aryl Ether as a Negishi Coupling Partner: An Approach for Constructing C-C Bonds under Mild Conditions. Chem. Eur. J. 2012, 18, 3482–3485; (k) Leiendecker, M.; Hsiao, C. C.; Guo, L.; Alandini, N.; Rueping, M. Metal-Catalyzed Dealkoxylative Caryl-Csp3 Cross-Coupling-Replacement of Aromatic Methoxy Groups of Aryl Ethers by Employing a Functionalized Nucleophile. Angew. Chem. Int. Ed. 2014, 53, 12912–12915; (l) Yang, Z. K.; Wang, D. Y.; Minami, H.; Ogawa, H.; Ozaki, T.; Saito, T.; Miyamoto, K.; Wang, C.; Uchiyama, M. Cross-Coupling of Organolithium with Ethers or Aryl Ammonium Salts by C-O or C-N Bond Cleavage. Chem. Eur. J. 2016, 22, 15693–15699; (m) Zarate, C.; Manzano, R.; Martin, R. Ipso- Borylation of Aryl Ethers via Ni-Catalyzed C-OMe Cleavage. J. Am. Chem. Soc. 2015, 137, 6754–6757; (n) Tobisu, M.; Takahira, T.; Ohtsuki, A.; Chatani, N. Nickel-Catalyzed Alkynylation of Anisoles via C-O Bond Cleavage. Org. Lett. 2015, 17, 680–683. (o) Morioka, T.; Nishizawa, A.; Nakamura, K.; Tobisu, M.; Chatani, N. Nickel-Catalyzed Cross-Coupling of Anisole Derivatives with Trimethylaluminum through the Cleavage of Carbon-Oxygen Bonds. Chem. Lett. 2015, 44, 1729–1731; (p) Liu, X.; Hsiao, C. C.; Kalvet, I.; Leiendecker, M.; Guo, L.; Schoenebeck, F.; Rueping, M. Lewis Acid Assisted Nickel-Catalyzed Cross-Coupling of Aryl Methyl Ethers by C-O Bond-Cleaving Alkylation: Prevention of Undesired β-Hydride Elimination. Angew. Chem. Int. Ed. 2016, 55, 6093–6098; (q) Nakamura, K.; Tobisu, M.; Chatani, N. Nickel-Catalyzed Formal Homocoupling of Methoxyarenes for the Synthesis of Symmetrical Biaryls via C-O Bond Cleavage. Org. Lett. 2015, 17, 6142–6145; (r) Alvarez-Bercedo, P.; Martin, R. Ni-Catalyzed Reduction of Inert C-O Bonds: A New Strategy for Using Aryl Ethers as Easily Removable Directing Groups. J. Am. Chem. Soc. 2010, 132, 17352–17353.
- 11 Wenkert, E.; Michelotti, E. L.; Swingdell, C. S.; Tingoli, M. J. Transformation of Carbon-Oxygen into Carbon-Carbon Bonds Mediated by Low-Valent Nickel Species. J. Org. Chem. 1984, 49, 4894.
- 12(a) Chen, G. J.; Huang, J.; Gao, L. X.; Han, F. S. Nickel-Catalyzed Cross-Coupling of Phenols and Arylboronic Acids through an in situ Phenol Activation Mediated by PyBroP. Chem. Eur. J. 2011, 17, 4038–4042; (b) Shi, C.; Aldrich, C. C. Efficient Pd-Catalyzed Coupling of Tautomerizable Heterocycles with Terminal Alkynes via C-OH Bond Activation Using PyBrOP. Org. Lett. 2010, 12, 2286–2289; (c) Kang, F. A.; Lanter, J. C.; Cai, C.; Sui, Z.; Murray, W. V. Direct Dehydrative Cross-Coupling of Tautomerizable Heterocycles with Alkynes via Pd/Cu-Catalyzed Phosphonium Coupling. Chem. Commun. 2010, 46, 1347–1349; (d) Mehta, V. P.; Modha, S. G.; Van der Eycken, E. V. Microwave-Assisted Palladium-Catalyzed Phosphonium Coupling of 2(1H)-Pyrazinones. J. Org. Chem. 2010, 75, 976–979; (e) Kang, F. A.; Sui, Z.; Murray, W. V. Pd-Catalyzed Direct Arylation of Tautomerizable Heterocycles with Aryl Boronic Acids via C-OH Bond Activation Using Phosphonium Salts. J. Am. Chem. Soc. 2008, 130, 11300–11302; (f) Kang, F. A.; Sui, Z.; Murray, W. V. Phosphonium Coupling in the Direct Bond Formations of Tautomerizable Heterocycles via C-OH Bond Activation. Eur. J. Org. Chem. 2009, 461–479.
- 13(a) Iranpoor, N.; Panahi, F. Nickel-Catalyzed One-Pot Deoxygenation and Reductive Homocoupling of Phenols via C-O Activation Using TCT Reagent. Org. Lett. 2015, 17, 214–217; (b) Li, Z.; Zhang, S. L.; Fu, Y.; Guo, Q. X.; Liu, L. Mechanism of Ni-Catalyzed Selective C-O Bond Activation in Cross-Coupling of Aryl Esters. J. Am. Chem. Soc. 2009, 131, 8815–8823; (c) Ueno, S.; Mizushima, E.; Chatani, N.; Kakiuchi, F. Direct Observation of the Oxidative Addition of the Aryl Carbon-Oxygen Bond to a Ruthenium Complex and Consideration of the Relative Reactivity between Aryl Carbon-Oxygen and Aryl Carbon-Hydrogen Bonds. J. Am. Chem. Soc. 2006, 128, 16516–16517.
- 14 Zhao, Y. L.; Wu, G. J.; Han, F. S. Ni-Catalyzed Construction of C-P Bonds from Electron-Deficient Phenols via the In-Situ Aryl C-O Activation by PyBroP. Chem. Commun. 2012, 48, 5868–5870.
- 15 Iranpoor, N.; Panahi, F. Direct Nickel-Catalyzed Amination of Phenols via C-O Bond Activation using 2,4,6-Trichloro-1,3,5-triazine (TCT) as Reagent. Adv. Synth. Catal. 2014, 356, 3067–3073.
- 16 Khakyzadeh, V.; Rostami, A.; Veisi, H.; Shaghasemi, B. S.; Reimhult, E.; Luque, R.; Xia, Y.; Darvishi, S. Direct C-S Bond Formation via C-O Bond Activation of Phenols in a Crossover Pd/Cu Dual-Metal Catalysis System. Org. Biomol. Chem. 2019, 17, 4491–4497.
- 17 Barker, R. S. Preparation of Aminated Benzenes from Hydroxy Benzenes. US3272865, 1966.
- 18 Becker, M.; Russell, J. L. Chem. Eng. (N. Y.) 1973, 80, 42–43.
- 19 McKechnie, I.; Bayer, F.; Drennan, J. Chem. Eng. (N. Y.) 1980, 29, 26–27.
- 20 Mitchell, B.; Sargis, K. Process for the Production of Organic Amines. US3860650, 1975.
- 21 Ono, Y.; Ishida, H. Amination of Phenols with Ammonia over Palladium Supported on Alumina. J. Catal. 1981, 72, 121–128.
- 22 Chang, C. D.; Lang, W. H. Aniline or Substituted Aniline from Phenol or Phenolic Compounds. EP62542A1, 1982.
- 23 Chang, C. D.; Perkins, P. D. 2-Methylpyridine from Benzamine. A Novel Rearrangement Catalysed by Zeolite. Zeolites 1983, 3, 298–299.
- 24 Yasuhara, M.; Matsunaga, F. Preparation of Anilines. US4987260, 1991.
- 25 Mori, Y.; Noro, H.; Hara, Y.; Washama, T. Preparation of Aromatic Amines from Phenols. JP06184062A, 1994.
- 26 Ma, J.; Wang, L.; Chen, L.; Dong, X.; Wang, L. Bentonite-Based Amination Reaction Catalyst Preparation Method. CN103418373A, 2013.
- 27 Deger, T. E.; Goshorn, R. H. Novel Catalytic Process for Preparation of Mercaptans by Reaction of H2S with Alcohols or Ethers. US3035097, 1962.
- 28 Sakurada, A.; Hirowatari, N. Aromatic Sulfur Compounds. JP55036409A, 1980.
- 29 Matsumura, Y. Preparation of Thiols with High Selectivity from Hydroxy Compounds and Hydrogen Sulfide. JP2011126873A, 2011.
- 30 Hamada, H.; Yamamoto, M.; Kuwahara, Y.; Matsuzaki, T.; Wakabayashi, K. The Co-Amination of Phenol and Cyclohexanol with Palladium-on-carbon Catalyst in the Liquid Phase. An Application of a Hydrogen-transfer Reaction. Bull. Chem. Soc. Jpn. 1985, 58, 1551–1555.
- 31 Chen, Z.; Zeng, H.; Girard, S. A.; Wang, F.; Chen, N.; Li, C. J. Formal Direct Cross-Coupling of Phenols with Amines. Angew. Chem. Int. Ed. 2015, 54, 14487–14491.
- 32 Chen, Z.; Zeng, H.; Gong, H.; Wang, H.; Li, C. J. Palladium-Catalyzed Reductive Coupling of Phenols with Anilines and Amines: Efficient Conversion of Phenolic Lignin Model Monomers and Analogues to Cyclohexylamines. Chem. Sci. 2015, 6, 4174–4178.
- 33 Li, J. S.; Qiu, Z.; Li, C. J. Palladium-Catalyzed Synthesis of N-Cyclohexyl Anilines from Phenols with Hydrazine or Hydroxylamine via N-N/O- Cleavage. Adv. Synth. Catal. 2017, 359, 3648–3653.
- 34 Dominguez-Huerta, A.; Perepichka, I.; Li, C.-J. Direct Synthesis of Diphenylamines from Phenols and Ammonium Formate Catalyzed by Palladium. ChemSusChem 2019, 12, 2999–3002.
- 35 Amant, A. H. St.; Frazier, C. P.; Newmeyer, B.; Fruehauf, K. R.; Read de Alaniz, J. Direct Synthesis of Anilines and Nitrosobenzenes from Phenols. Org. Biomol. Chem. 2016, 14, 5520–5524.
- 36 Cuypes, T.; Tomkins, P.; Vos, D. E. D. Direct Liquid-Phase Phenol-to- Aniline Amination Using Pd/C. Catal. Sci. Technol. 2018, 8, 2519–2523.
- 37 Luo, Z. C.; Zheng, Z. X.; Wang, Y. C.; Sun, G.; Jiang, H.; Zhao, C. Hydrothermally Stable Ru/HZSM-5-catalyzed Selective Hydrogenolysis of Lignin-derived Substituted Phenols to Bio-arenes in Water. Green Chem. 2016, 18, 5845–5858.
- 38 Huang, Y. B.; Yan, L.; Chen, M. Y.; Guo, Q. X.; Fu, Y. Selective Hydrogenolysis of Phenols and Phenyl Ethers to Arenes through Direct C-O Cleavage over Ruthenium-Tungsten Bifunctional Catalysts. Green Chem. 2015, 17, 3010–3017.
- 39 Xu, L. J.; Han, Z.; Zhang, Y.; Fu, Y. In Situ Synthesis of Molybdenum oxide N-doped Carbon from Biomass for Selective Vapor Phase Hydrodeoxygenation of Lignin-Derived Phenols Under H2 Atmosphere. RSC Adv. 2016, 6, 108217–108228.
- 40 Zhang, C.; Jia, C. H.; Cao, Y.; Yao, Y.; Xie, S. Q.; Zhang, S. C.; Lin, H. F. Water-Assisted Selective Hydrodeoxygenation of Phenol to Benzene over the Ru Composite Catalyst in the Biphasic Process. Green Chem. 2019, 21, 1668–1679.
- 41 Jia, X.; An, W.; Wang, Z. M.; Zhou, J. W. Effect of Doped Metals on Hydrodeoxygenation of Phenol over Pt-Based Bimetallic Alloys: Caryl-OH Versus CaliphaticH-OH Bond Scission. J. Phys. Chem. C 2019, 123, 16873–16882.
- 42 Zhou, J. W.; An, W.; Wang, Z. M.; Jia, X. Hydrodeoxygenation of Phenol over Ni-Based Bimetallic Single-Atom Surface Alloys: Mechanism, Kinetics and Descriptor. Catal. Sci. Technol. 2019, 9, 4314–4326.
- 43 Xu, H.; Yu, B.; Zhang, H.; Zhao, Y.; Yang, Z.; Xu, J.; Han, B.; Liu, Z. Reductive Cleavage of Inert Aryl C-O Bonds to Produce Arenes. Chem. Commun. 2015, 51, 12212–12215.
- 44 Kusumoto, S.; Nozaki, K. Direct and Selective Hydrogenolysis of Arenols and Aryl Methyl Ethers. Nat. Commun. 2015, 6, 6296–6302.
- 45 Shi, W. J.; Li, X. L.; Li, Z. W.; Shi, Z. J. Nickel Catalyzed Reduction of Arenols Under Mild Conditions. Org. Chem. Front. 2016, 3, 375–379 .
- 46 Ohgi, A.; Nakao, Y. Selective Hydrogenolysis of Arenols with Hydrosilanes by Nickel Catalysis. Chem. Lett. 2016, 45, 45–47.
- 47 Wang, X. Y.; Leng, J.; Wang, S. M.; Asiri, A. M.; Marwani, H. M.; Qin, H. L. A Facile and Mild Pd-Catalyzed One-Pot Process for Direct Hydrodeoxygenation (HDO) Phenols to Arenes through a ArOSO2F Intermediates Transformation. Tetrahedron Lett. 2017, 58, 2340–2343.
- 48 Yu, D. G.; Li, B. J.; Zheng, S. F.; Guan, B. T.; Wang, B. Q.; Shi, Z. J. Direct Application of Phenolic Salts to Nickel-Catalyzed Cross-Coupling Reactions with Aryl Grignard Reagents. Angew. Chem. Int. Ed. 2010, 49, 4566–4570.
- 49 Yu, D. G.; Shi, Z. J. Mutual Activation: Suzuki-Miyaura Coupling through Direct Cleavage of the sp2 C-O Bond of Naphtholate. Angew. Chem. Int. Ed. 2011, 50, 7097–7100.
- 50(a) Cao, Z. C.; Luo, F. X.; Shi, W. J.; Shi, Z. J. Direct Borylation of Benzyl Alcohol and Its Analogues in the Absence of Bases. Org. Chem. Front. 2015, 2, 1505–1510; (b) Shi, W. J.; Shi, Z. J. Methylation of Arenols through Ni-catalyzed C-O Activation with Methyl Magnesium Bromide. Chin. J. Chem. 2018, 36, 183–186; (c) Cao, Z. C.; Xu, P. L.; Luo, Q. Y.; Li, X. L.; Yu, D. G.; Fang, H. Y.; Shi, Z. J. Conversion of Carbonyl Compounds to Olefins Via Enolate Intermediate. Chin. J. Chem. 2019, 37, 781–785.
- 51 Lee, D. H.; Kwon, K. H.; Yi, C. S. Selective Catalytic C-H Alkylation of Alkenes with Alcohols. Science 2011, 333, 1613–1616.
- 52 Yu, D. G.; Wang, X.; Zhu, R. Y.; Luo, S.; Zhang, X. B.; Wang, B. Q.; Wang, L.; Shi, Z. J. Direct Arylation/Alkylation/Magnesiation of Benzyl Alcohols in the Presence of Grignard Reagents via Ni-, Fe-, or Co-Catalyzed sp3 C-O Bond Activation. J. Am. Chem. Soc. 2012, 134, 14638–14641.
- 53 Cao, Z.-C.; Yu, D.-G.; Zhu, R.-Y.; Wei, J.-B.; Shi, Z.-J. Direct Cross-Coupling of Benzyl Alcohols to Construct Diarylmethanes via Palladium Catalysis. Chem. Commun. 2015, 51, 2683–2686.




