Ullmann-Ma Reaction: Development, Scope and Applications in Organic Synthesis†
Corresponding Author
Qian Cai
College of Pharmacy, Jinan University, No. 601 Huangpu Avenue West, Guangzhou, Guangdong, 510632 China
E-mail: [email protected]Search for more papers by this authorWei Zhou
College of Pharmacy, Jinan University, No. 601 Huangpu Avenue West, Guangzhou, Guangdong, 510632 China
Search for more papers by this authorCorresponding Author
Qian Cai
College of Pharmacy, Jinan University, No. 601 Huangpu Avenue West, Guangzhou, Guangdong, 510632 China
E-mail: [email protected]Search for more papers by this authorWei Zhou
College of Pharmacy, Jinan University, No. 601 Huangpu Avenue West, Guangzhou, Guangdong, 510632 China
Search for more papers by this author† Dedicated to the 70th Anniversary of Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences.
Summary
Copper-catalyzed cross-couplings of aryl halides and nucleophiles, traditionally called Ullmann-type coupling reactions, were initially reported by Ullmann et al. from 1901—1929. A seminal report in 1998 by Ma et al. from Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences revealed an accelerating effect caused by amino acids, which brought Ullmann-type coupling reactions into a ligand-accelerating era. From 1999 to the first 10 years of 2000s, the first-generation ligands were developed by many researchers and promoted Ullmann-type coupling reactions of aryl iodides and bromides under relatively mild conditions. Amino acid ligands, developed by Ma and coworkers, are one class of the most important first-generation ligands. In the second 10 years of 2000s, Ma et al. led the discovery of second-generation ligands for copper-catalyzed cross-coupling reactions. Two great breakthroughs have been realized by using second-generation oxalic diamide and related amide ligands, with aryl chlorides as general coupling partner and with low catalyst loadings. Now copper-catalyzed cross coupling reactions of aryl halides and nucleophiles with amino acids or oxalic diamides and related amides as ligands are recognized as Ullmann-Ma reactions and have found extensive applications in organic synthesis.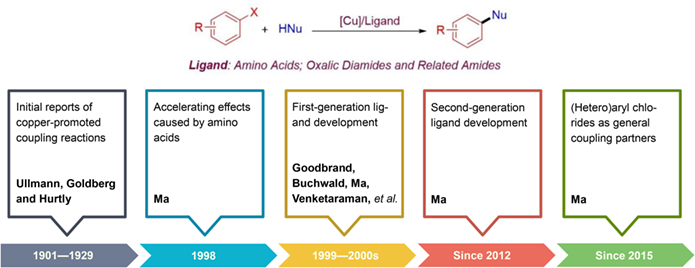
References
- 1(a) Magano, J.; Dunetz, J. R. Transition Metal-Catalyzed Couplings in Process Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2013.
- 2
Evano, G.; Blanchard, N. Copper-Mediated Cross-Coupling Reactions, Wiley, Hoboken, 2013.
10.1002/9781118690659 Google Scholar
- 3 Ullmann, F.; Bielecki, J. Ueber Synthesen in der Biphenylreihe. Chem. Ber. 1901, 34, 2174–2185; (b) Ullmann, F. Ueber eine neue Bildungsweise von Diphenylaminderivaten. Chem. Ber. 1903, 36, 2382–2384; (c) Ullmann, F.; Sponagel, P. Ueber die Phenylirung von Phenolen. Chem. Ber. 1905, 38, 2211–2212.
- 4 Goldberg, I. Ueber Phenylirungen bei Gegenwart von Kupfer als Katalysator. Chem. Ber. 1906, 39, 1691–1692.
- 5 Hurtley, W. R. H. Replacement of Halogen α-Bromobenzoic Acid. J. Chem. Soc. 1929, 1870–1873.
- 6For selected reviews: (a) Dai, L. Ullmann Reaction, A Centennial Memory and Recent Renaissance-Related Formation of Carbon- Heteroatom Bond. Prog. Chem. 2018, 30, 1257–1297; (b) Bhunia, S.; Pawar, G. G.; Kumar, S. V.; Jiang, Y.; Ma, D. Selected Copper-Based Reactions for C-N, C-O, C-S and C-C Bond Formation. Angew. Chem. Int. Ed. 2017, 56, 16136–16179; (c) Sambiagio, C.; Marsden, S. P.; Blacker, M. A.; McGowan, P. C. Copper Catalysed Ullmann Type Chemistry: From Mechanistic Aspects to Modern Development. Chem. Soc. Rev. 2014, 43, 3525; (d) Rao, H.; Fu, H. Copper-Catalyzed Coupling Reactions. Synlett 2011, 745–769; (e) Surry, D. S.; Buchwald, S. L. Diamine Ligands in Copper-Catalyzed Reactions. Chem. Sci. 2010, 1, 13–31; (f) Ma, D.; Cai, Q. Copper/Amino Acid Catalyzed Cross- Couplings of Aryl and Vinyl Halides with Nucleophiles. Acc. Chem. Res. 2008, 41, 1450–1460; (g) Beletskaya, I.; Cheprakov, A. V. Copper in Cross-Coupling Reactions The Post-Ullmann Chemistry Coord. Chem. Rev. 2004, 248, 2337–2364; (h) Ley, S. V.; Thomas, A. W. Modern Synthetic Methods for Copper-Mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S Bond Formation. Angew. Chem. Int. Ed. 2003, 42, 5400–5449.
- 7For selected reviews: (a) Maaliki, C.; Thiery, E.; Thibonnet, J. Emergence of Copper-Mediated Formation of C-C Bonds. Eur. J. Org. Chem. 2017, 2, 209–228; (b) Okano, K.; Tokuyama, H.; Fukuyama, T. Copper-Mediated Aromatic Amination Reaction and Its Application to the Total Synthesis of Natural Products. Chem. Commun. 2014, 50, 13650–13663; (c) Lin, H.; Sun, D. Recent Synthetic Developments and Applications of the Ullmann Reaction. A Review. Org. Prep. Proceed. Int. 2013, 45, 341–394; (d) Cacchi, S.; Fabrizi, G.; Goggiamani, A. Copper Catalysis in the Construction of Indole and Benzo[b]furan Rings. Org. Biomol. Chem. 2011, 9, 641–652; (e) Evano, G.; Toumi, M.; Coste, A. Copper-Catalyzed Cyclization Reactions for the Synthesis of Alkaloids. Chem. Commun. 2009, 4166–4175; (f) Evano, G.; Blanchard, N.; Toumi, M. Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis. Chem. Rev. 2008, 108, 3054–3131.
- 8 Huang, P. Q. Organic Name Reactions, Reagents and Rules, 2nd Ed., Chemical Industry Press, Beijing, 2019, pp. 324–332.
- 9 Ma, D.; Zhang, Y.; Yao, J.; Wu, S.; Tao, F. Accelerating Effect Induced by the Structure of α-Amino Acid in the Copper-Catalyzed Coupling Reaction of Aryl Halides with α-Amino Acids. Synthesis of Benzolactam-V8. J. Am. Chem. Soc. 1998, 120, 12459–12467.
- 10(a) Ma, D.; Xia, C. CuI-Catalyzed Coupling Reaction of β-Amino Acids or Esters with Aryl Halides at Temperature Lower than that Employed in the Normal Ullmann Reaction. Facile Synthesis of SB-214857. Org. Lett. 2001, 3, 2583–2586; (b) Ma, D.; Xia, C.; Jiang, J.; Zhang, J. First Total Synthesis of Martinellic Acid, a Naturally Occurring Bradykinin Receptor Antagonist. Org. Lett. 2001, 3, 2189–2191; (c) Ma, D.; Xia, C.; Jiang, J.; Zhang, J.; Tang, W. Aromatic Nucleophilic Substitution or CuI-Catalyzed Coupling Route to Martinellic Acid. J. Org. Chem. 2003, 68, 442–451.
- 11(a) Ma, D.; Cai, Q.; Zhang, H. Mild Method for Ullmann Coupling Reaction of Amines and Aryl Halides. Org. Lett. 2003, 5, 2453–2455;
(b) Ma, D.; Cai, Q. L-Proline Promoted Ullmann Type Coupling Reactions of Aryl Iodides with Indoles, Pyrroles, Imidazoles or Pyrazoles. Synlett 2004, 128–130;
10.1055/s-2003-44995 Google Scholar(c) Zhang, H.; Cai, Q.; Ma, D. Amino Acid Promoted CuI-Catalyzed C-N Bond Formation between Aryl Halides and Amines or N-Containing Heterocycles, J. Org. Chem. 2005, 70, 5164–5173.
- 12 Xing, H.; Zhang, Y.; Lai, Y.; Jiang, Y.; Ma, D. Synthesis of Symmetric and Unsymmetrical N,N’-Diaryl Guanidines via Copper/N-Methylglycine-Catalyzed Arylation of Guanidine Nitrate. J. Org. Chem. 2012, 77, 5449–5453.
- 13 Zhu, W.; Ma, D. Synthesis of Aryl Azides and Vinyl Azides via Proline Promoted CuI-Catalyzed Coupling reactions. Chem. Commun. 2004, 888–889.
- 14(a) Xie, X.; Cai, G.; Ma, D. CuI/L-Proline-Catalyzed Coupling Reactions of Aryl Halides with Activated Methylene Compounds. Org. Lett. 2005, 7, 4693–4695; (b) Lu, B.; Ma, D. Assembly of 3-Acyloxindoles via CuI/L-Proline-Catalyzed Intramolecular Arylation of β-keto Amides. Org. Lett. 2006, 8, 6115–6118.
- 15 Zhu, W.; Ma, D. Synthesis of Aryl Sulfones via L-Proline Promoted CuI-Catalyzed Coupling Reaction of Aryl Halides with Sulfinic Acid Salts. J. Org. Chem. 2005, 70, 2696–2700.
- 16 Jiang, L.; Lu, X.; Zhang, H.; Jiang, Y.; Ma, D. CuI/4-Hydro-L-Proline as a More Effective Catalytic System for Coupling of Aryl Bromides with N-Boc Hydrazine and Aqueous Ammonia. J. Org. Chem. 2009, 74, 4542–4546.
- 17(a) Ma, D.; Cai, Q. N,N-Dimethylglycine Promoted Ullmann Coupling Reaction of Phenols and Aryl Halides. Org. Lett. 2003, 5, 3799–3802; (b) Cai, Q.; He, G.; Ma, D. Mild and Non-Racemization Conditions for Ullmann-Type Diaryl Ether Formation between Aryl Halides and Tyrosine Derivatives. J. Org. Chem. 2006, 71, 5268–5273.
- 18 Zhang, H.; Ma, D.; Cao, W. N,N-Dimethylglycine-Promoted Ullmann- Type Coupling Reactions of Aryl Iodides with Aliphatic Alcohols. Synlett 2007, 243–246.
- 19(a) Pan, X.; Cai, Q.; Ma, D. CuI/N,N-Dimethylglycine-Catalyzed Coupling of Vinyl Halides with Amides or Carbamates. Org. Lett. 2004, 6, 1809–1812; (b) Li, J.; Zhang, Y.; Jiang, Y.; Ma, D. CuI/N,N-Dimethylglycine-Catalyzed Synthesis of N-Aryloxazolidinones from Aryl Bromides. Tetrahedron Lett. 2012, 53, 3981–3983.
- 20(a) Ma, D.; Liu, F. CuI-Catalyzed Coupling Reaction of Aryl Halides with Terminal Alkynes in the Absence of Palladium and Phosphine. Chem. Commun. 2004, 1934–1935; (b) Ma, D.; Liu, F. Assembly of Conjugated Enynes and Substituted Indoles via CuI/Amino Acid-Catalyzed Coupling of 1-Alkynes with Vinyl Iodides and 2-Bromotrifluoroacetanilides. J. Org. Chem. 2007, 72, 4844–4850.
- 21 Gan, J.; Ma, D. Synthesis of 1,5-Benzothiazepine Dipeptide Mimetics via Two CuI-Catalyzed Cross Coupling Reactions. Org. Lett. 2009, 11, 2788–2790.
- 22 Cai, Q.; Zou, B.; Ma, D. Mild Ullmann-Type Biaryl Ether Formation Reaction via Combination of ortho-Substituent and Ligand Effects. Angew. Chem. Int. Ed. 2006, 45, 1276–1279.
- 23 Xie, X.; Chen, Y.; Ma, D. Enantioselective Arylation of 2-Methylacetoacetates Catalyzed by CuI/trans-4-Hydroxy-L-Proline at Low Reaction Temperature. J. Am. Chem. Soc. 2006, 128, 16050–16051.
- 24 Zou, B.; Yuan, Q.; Ma, D. Synthesis of 1,2-Disubstituted Benzimidazoles by a Cu-Catalyzed Cascade Aryl Amination/Condensation Process. Angew. Chem. Int. Ed. 2007, 46, 2598–2601.
- 25(a) Zou, B.; Yuan, Q.; Ma, D. Cascade Coupling/Cyclization Process to N-Substituted 1,3-Dihydrobenzimidazol-2-ones. Org. Lett. 2007, 9, 4291–4294; (b) Diao, X.; Wang, Y.; Jiang, Y.; Ma, D. Assembly of Substituted 1H-Benzimidazoles and 1,3-Dihydrobenimidazol-2-ones via CuI/L-Proline Catalyzed Coupling of Aqueous Ammonia with 2-Iodoacetanilides and 2-Iodophenylcarbamates. J. Org. Chem. 2009, 74, 7974–7977.
- 26 Chen, Y.; Xie, X.; Ma, D. Facile Access to Polysubstituted Indoles via a Cascade Cu-Catalyzed Arylation-Condensation Process. J. Org. Chem. 2007, 72, 9329–9334.
- 27 Chen, Y.; Wang, Y.; Sun. Z.; Ma, D. Elaboration of 2-(Trifluoromethyl)indoles via a Cascade Coupling/Condensation Deacylation Process. Org. Lett. 2008, 10, 625–628.
- 28 Ma, D.; Xie, S.; Xue, P.; Zhang, X.; Dong, J.; Jiang, Y. Efficient and Economical Access to Substituted Benzothiazoles: Copper-Catalyzed Coupling of 2-Haloanilides with Metal Sulfides and Subsequent Condensation. Angew. Chem. Int. Ed. 2009, 48, 4222–4225.
- 29 Yuan, Q.; Ma, D. A One-Pot Coupling/Hydrolysis/Condensation Process to Pyrrolo[1,2-a]quinoxaline. J. Org. Chem. 2008, 73, 5159–5162.
- 30 Xu, L.; Jiang, Y.; Ma, D. Synthesis of Tetrahydropyrrolo[1,2-a]quinoxalines and Tetrahydropyrido[1,2-a]quinoxalines via One-Pot CuI-Catalyzed Aryl Amination-Hydrolysis-Condensation Process. Synlett 2010, 2285–2288.
- 31 Beletskaya, I. P.; Cheprakov, A. V. The Complementary Competitors: Palladium and Copper in C-N Cross-Coupling Reactions. Organometallics 2012, 31, 7753–7808.
- 32(a) Gildner, P. G.; Colacot, T. J. Reactions of the 21st Century: Two Decades of Innovative Catalyst Design for Palladium-Catalyzed Cross-Couplings. Organometallics 2015, 34, 5497–5508; (b) Bruneau, A.; Roche, M.; Alami, M.; Messaoudi, S. 2-Aminobiphenyl Palladacycles: The “Most Powerful” Precatalysts in C-C and C-Heteroatom Cross-Couplings. ACS Catal. 2015, 5, 1386–1396; (c) Crawford, S. M.; Lavery, C. B.; Stradiotto, M. Chem. Eur. J. 2013, 19, 16760–16771; (d) Surry, D. S.; Buchwald, S. L. Biaryl Phosphane Ligands in Palladium- Catalyzed Amination. Angew. Chem. Int. Ed. 2008, 47, 6338–6361; (e) Hartwig, F. Evolution of a Fourth Generation Catalyst for the Amination and Thioetherification of Aryl Halides. Acc. Chem. Res. 2008, 41, 1534–1544; (f) Kantchev, E. A. B.; O'Brien, C. J.; Organ, M. G. Palladium Complexes of N-Heterocyclic Carbenes as Catalysts for Cross- Coupling Reactions—A Synthetic Chemist's Perspective. Angew. Chem. Int. Ed. 2007, 46, 2768–2813.
- 33 Zhang, Y.; Yang, X.; Yao, Q.; Ma, D. CuI/DMPAO-Catalyzed N-Arylation of Acyclic Secondary Amines. Org. Lett. 2012, 14, 3056–3059.
- 34(a) Yang, X.; Zhang, Y.; Ma, D. Synthesis of Aryl Carbamates via Copper-Catalyzed Coupling of Aryl Halides with Potassium Cyanate. Adv. Synth. Catal. 2012, 354, 2443–2446; (b) Kumar, S. V.; Ma, D. Synthesis of N-(Hetero)aryl Carbamates via CuI/MNAO Catalyzed Cross- Coupling of (Hetero)aryl Halides with Potassium Cyanate in Alcohols. J. Org. Chem. 2018, 83, 2706–2713.
- 35 Yin, H.; Chen, B.; Zhang, X.; Yang, X.; Zhang, Y.; Jiang, Y.; Ma, D. Assembly of N,N-Disubstituted-N’-arylureas via a Copper-Catalyzed One-Pot Three-Component Reaction of Aryl Bromides, Potassium Cyanate, and Secondary Amines. Tetrahedron 2013, 69, 5326–5330.
- 36 Zhou, W.; Fan, M.; Yin, J.; Jiang, Y.; Ma, D. CuI/Oxalic Diamide Catalyzed Coupling Reaction of (Hetero)Aryl Chlorides and Amines. J. Am. Chem. Soc. 2015, 137, 11942–11945.
- 37 Bhunia, S.; Kumar, S. V.; Ma, D. N,N’-Bisoxalamides Enhance the Catalytic Activity in Cu-Catalyzed Coupling of (Hetero)Aryl Bromides with Anilines and Secondary Amines. J. Org. Chem. 2017, 82, 12603–12612.
- 38 Fan, M.; Zhou, W.; Jiang, Y.; Ma, D. Assembly of Primary (Hetero)Arylamines via CuI/Oxalic Diamide-Catalyzed Coupling of Aryl Chlorides and Ammonia. Org. Lett. 2015, 17, 5934–5937.
- 39(a) Gao, J.; Bhunia, S.; Wang, K.; Gan, L.; Xia, S.; Ma, D. Discovery of N-(Naphthalen-1-yl)-N’-alkyl Oxalamide Ligands Enables Cu-Catalyzed Aryl Amination with High Turnovers. Org. Lett. 2017, 19, 2809–2812; (b) Kumar, S. V.; Ma, D. Synthesis of Aryl Hydrazines via CuI/BMPO Catalyzed Cross-Coupling of Aryl Halides with Hydrazine Hydrate in Water. Chin. J. Chem. 2018, 36, 1003–1006.
- 40 De, S.; Yin, J.; Ma, D. Copper-Catalyzed Coupling Reactions of (Hetero)Aryl Chlorides and Amides. Org. Lett. 2017, 17, 4864–4874.
- 41 Pawar, G. G.; Wu, H.; De, S.; Ma, D. Copper(I) Oxide/N,N’-Bis[(2-furyl)methyl]oxalamide-Catalyzed Coupling of (Hetero)aryl Halides and Nitrogen Heterocycles at Low Catalytic Loading. Adv. Synth. Catal. 2017, 359, 1631–1636.
- 42 Chen, Z.; Ma, D. Cu/N,N’-Dibenzyloxalamide-Catalyzed N-Arylation of Heteroanilines. Org. Lett. 2019, 21, 6874–6878.
- 43 Fan, M.; Zhou, W.; Jiang, Y.; Ma, D. CuI/Oxalamide Catalyzed Couplings of (Hetero)aryl Chlorides and Phenols for Diaryl Ether Formation. Angew. Chem. Int. Ed. 2016, 55, 6211–6215.
- 44 Zhai, Y.; Chen, X.; Zhou, W.; Fan, M.; Lai, Y.; Ma, D. Copper-Catalyzed Diaryl Ether Formation from (Hetero)aryl Halides at Low Catalytic Loadings. J. Org. Chem. 2017, 82, 4964–4969.
- 45 Xia, S.; Gan, L.; Wang, K.; Li, Z.; Ma, D. Copper-Catalyzed Hydroxylation of (Hetero)aryl Halides under Mild Conditions. J. Am. Chem. Soc. 2016, 138, 13493–13496.
- 46 Chen, Z.; Jiang, Y.; Zhang, L.; Guo, Y.; Ma, D. Oxalic Diamides and tert-Butoxide: Two Types of Ligands Enabling Practical Access to Alkyl Aryl Ethers via Cu-Catalyzed Coupling Reaction. J. Am. Chem. Soc. 2019, 141, 3541–3549.
- 47 Ma, D.; Niu, S.; Zhao, J.; Jiang, X.; Jiang, Y.; Zhang, X.; Sun, T. A New Class of Amide Ligands Enable Cu-Catalyzed Coupling of Sodium Methanesulfinate with (Hetero)aryl Chlorides. Chin. J. Chem. 2017, 35, 1661–1664.
- 48 Zhao, J.; Niu, S.; Jiang, X.; Jiang, Y.; Zhang, X.; Sun, T.; Ma, D. A Class of Amide Ligands Enable Cu-Catalyzed Coupling of (Hetero)aryl Halides with Sulfinic Acid Salts under Mild Conditions. J. Org. Chem. 2018, 83, 6589–6598.
- 49 Devi, C. L.; Yesudas, K.; Makarov, N. S.; Rao, V. J.; Bhanuprakash, K.; Perry, J. W. Fluorenylethynylpyrene Derivatives with Strong Two- Photon Absorption: Influence of Substituents on Optical Properties. J. Mater. Chem. C 2015, 3, 3730–3744.
- 50 Suzuki, K.; Kubo, S.; Shizu, K.; Fukushima, T.; Wakamiya, A.; Murata, Y.; Adachi, C.; Kaji, H. Triarylboron-Based Fluorescent Organic Light- Emitting Diodes with External Quantum Efficiencies Exceeding 20%. Angew. Chem. Int. Ed. 2015, 54, 15231–15235.
- 51 Jin, S.; Kato, S.; Nakamura, Y. Synthesis, Self-association, and Anion Recognition of Conjugated Macrocycles Composed of Carbazole and Triazolium Moieties. Chem. Lett. 2016, 45, 869–871.
- 52 Ban, X.; Sun, K.; Sun, Y.; Huang, B.; Jiang, W. Enhanced Electron Affinity and Exciton Confinement in Exciplex-Type Host: Power Efficient Solution-Processed Blue Phosphorescent OLEDs with Low Turn-on Voltage. ACS Appl. Mater. Interfaces 2016, 8, 2010–2016.
- 53 Chan, C.-Y.; Wong, Y.-C.; Chan, M.-Y.; Cheung, S.-H.; So, S.-K.; Yam, V. W.-W. Hole-Transporting Spirothioxanthene Derivatives as Donor Materials for Efficient Small-Molecule-Based Organic Photovoltaic Devices. Chem. Mater. 2014, 26, 6585–6594.
- 54 Zhou, H.; Zhao, Y.; Gao, G.; Li, S.; Lan, J.; You, J. Highly Selective Fluorescent Recognition of Sulfate in Water by Two Rigid Tetrakisimidazolium Macrocycles with Peripheral Chains. J. Am. Chem. Soc. 2013, 135, 14908–14911.
- 55 Huang, Y.; Wu, D.; Huang, J.; Guo, Q.; Li, J.; You, J. Use of the Wilkinson Catalyst for the ortho-C-H Heteroarylation of Aromatic Amines: Facile Access to Highly Extended π-Conjugated Heteroacenes for Organic Semiconductors. Angew. Chem. Int. Ed. 2014, 53, 12158–12162.
- 56 Hu, J. J.; Wong, N.-K.; Lu, M.-Y.; Chen, X.; Ye, S.; Zhao, A. Q.; Gao, P.; Kao, R. Y.-T.; Shen, J.; Yang, D. HKOCl-3: a Fluorescent Hypochlorous Acid Probe for Live-Cell and in vivo Imaging and Quantitative Application in Flow Cytometry and a 96-Well Microplate Assay. Chem. Sci. 2016, 7, 2094–2099.
- 57 Li, F.; Zhu, Y.-Z.; Zhang, S.-C.; Gao, H.-H.; Pan, B.; Zheng, J.-Y. Pyrrolo[3,2,1-kl]phenothiazine-based D-π-A Type Organic Dye for Efficient Dye-Sensitized Solar Cells. Dyes Pigm. 2017, 139, 292–299.
- 58 Haynes-Smith, J.; Diaz, I. Billingsly, K. L. Modular Total Synthesis of Protein Kinase Activator (-)-Indolactam V. Org. Lett. 2016, 18, 2008–2011.
- 59 Chacko, S.; Boshoff, H. I. M.; Singh, V.; Ferraris, D. M.; Gollapalli, D. R.; Zhang, M.; Lawson, A. P.; Pepi, M. J.; Joachimiak, A.; Rizzi, M.; Mizrahi, V.; Cuny, G. D.; Hedstrom, L. Expanding Benzoxazole-Based Inosine 5’-Monophosphate Dehydrogenase (IMPDH) Inhibitor Structure-Activity As Potential Antituberculosis Agents. J. Med. Chem. 2018, 61, 4739–4756.
- 60 Tang, G.; Wong, J. C.; Zhang, W.; Wang, Z.; Zhang, N.; Peng, Z.; Zhang, Z.; Rong, Y.; Li, S.; Zhang, M.; Yu, L.; Feng, T.; Zhang, X.; Wu, X.; Wu, J. Z.; Chen, L. Identification of a Novel Aminotetralin Class of HDAC6 and HDAC8 Selective Inhibitors. J. Med. Chem. 2014, 57, 8026–8034.
- 61Chobanian, H. R.; Guo, Y.; Liu, P.; Chioda, M. D.; Fung, S.; Lanza, T. J.; Chang, L.; Bakshi, R. K.; Dellureficio, J. P.; Hong, Q.; McLaughlin, M.; Belyk, K. M.; Krska, S. W.; Makarewicz, A. K.; Martel, E. J.; Leone, J. F.; Frey, L.; Karanam, B.; Madeira, M.; Alvaro, R.; Shuman, J.; Salituro, G.; Terebetski, J. L.; Jochnowitz, N.; Mistry, S.; McGowan, E.; Hajdu, R.; Rosenbach, M.; Abbadie, C.; Alexander, J. P.; Shiao, L.-L.; Sullivan, K. M.; Nargund, R. P.; Wyvratt, M. J.; Lin, L. S.; DeVita, R. J. Discovery of MK-4409, a Novel Oxazole FAAH Inhibitor for the Treatment of Inflammatory and Neuropathic Pain. ACS Med. Lett. 2014, 5, 717–721.
- 62 Genin, M. J.; Bueno, A. B.; Francisco, J. A.; Manninen, P. R.; Bocchinfuso, W. P.; Montrose-Rafizadeh, C.; Cannady, E. A.; Jones, T. M.; Stille, J. R.; Raddad, E.; Reidy, C.; Cox, A.; Michael, M. D.; Michael, L. F. Discovery of 6-(4-{[5-Cyclopropyl-3-(2,6-dichlorophenyl)isoxazol- 4-yl]methoxy}piperidin-1-yl)-1-methyl-1H-indole-3-carboxylic Acid: A Novel FXR Agonist for the Treatment of Dyslipidemia. J. Med. Chem. 2015, 58, 9768–9772.
- 63Huang, W.-S.; Liu, S.; Zou, D.; Thomas, M.; Wang, Y.; Zhou, T.; Romero, J.; Kohlmann, A.; Li, F.; Qi, J.; Cai, L.; Dwight, T. A.; Xu, Y.; Xu, R.; Dodd, R.; Toms, A.; Parillon, L.; Lu, X.; Anjum, R.; Zhang, S.; Wang, F.; Keats, J.; Wardwell, S. D.; Ning, Y.; Xu, Q.; Moran, L. E.; Mohemmad, Q. K.; Jang, H. G.; Clackson, T.; Narasimhan, N. I.; Rivera, V. M.; Zhu, X.; Dalgarno, D.; Shakespeare, W. C. Discovery of Brigatinib (AP26113), a Phosphine Oxide-Containing, Potent, Orally Active Inhibitor of Anaplastic Lymphoma Kinase. J. Med. Chem. 2016, 59, 4948–4964.
- 64 Chan, B. K.; Hanan, E. J.; Bowman, K. K.; Bryan, M. C.; Burdick, D.; Chan, E.; Chen, Y.; Clausen, S.; Vega, T. D.; Dotson, J.; Eigenbrot, C.; Elliot, R. L.; Heald, R. A.; Jackson, P. S.; Knight, J. D.; La, H.; Lainchbury, M. D.; Malek, S.; Purkey, H. E.; Schaefer, G.; Schemidt, S.; Seward, E. M.; Sideris, S.; Shao, L.; Wang, S.; Yeap, S. K.; Yen, I.; Yu, C.; Heffron, T. P. Discovery of a Noncovalent, Mutant-Selective Epitermal Growth Factor Receptro Inhibitor. J. Med. Chem. 2016, 59, 9080–9093.
- 65 Ko, K.; Kim, H.-J.; Ho, P.-S.; Lee, S. O.; Lee, J.-E.; Min, C.-R.; Kim, Y. C.; Yoon, J.-H.; Park, E.-J.; Kwon, Y.-J.; Yun, J.-H.; Yoon, D.-O.; Kim, J.-S.; Park, W.-S.; Oh, S.-S.; Song, Y.-M.; Cho, W.-K.; Morikawa, K.; Lee, K.-J.; Park, C.-H. Discovery of a Novel Highly Selective Histamine H4 Receptor Antagonist for the Treatment of Atopic Dermatitis. J. Med. Chem. 2018, 61, 2949–2961.
- 66 Colombo, R.; Wang, Z.; Han, J.; Balachandran, R.; Daghestani, H. N.; Camarco, D. P.; Vogt, A.; Day, B. W.; Mendel, D.; Wipf, P. Total Synthesis and Biological Evaluation of Tubulysin Analogues. J. Org. Chem. 2016, 81, 10302–10320.
- 67 Amour, A.; Barton, N.; Cooper, A. W. J.; Inglis, G.; Jamieson, C.; Luscombe, C. N.; Morrell, J.; Peace, S.; Perez, D.; Rowland, P.; Tame, C.; Uddin, S.; Vitulli, G.; Wellaway, N. Evolution of a Novel, Orally Bioavailable Series of PI3Kδ Inhibitors from an Inhaled Lead for the Treatment of Respiratory Disease. J. Med. Chem. 2016, 59, 7239–7251.
- 68 Corte, J. R.; Fang, T.; Osuna, H.; Pinto, D. J. P.; Rossi, K. A.; Myers, J. E. Jr.; Sheriff, S.; Lou, Z.; Zheng, J. J.; Harper, T. W.; Bozarth, J. M.; Wu, Y.; Luettgen, J. M.; Seiffert, D. A.; Decicco, C. P.; Wexler, R. R.; Quan, M. L. Structure-Based Design of Macrocyclic Factor Xia Inhibitors: Discovery of the Macrocyclic Amide Linker. J. Med. Chem. 2017, 60, 1060–1075.
- 69 Siegrist, R.; Pozzi, D.; Jacob, G.; Torrisi, C.; Colas, K.; Braibant, B.; Mawet, J.; Pfeifer, T.; de Kanter, R.; Roch, C.; Kessler, M.; Corminboeuf, O.; Bezençon, O. Structure-Activity Relationship, Drug Metabolism and Pharmacokinetics Properties Optimization, and in vivo Studies of New Brain Penetrant Triple T-Type Calcium Channel Blockers. J. Med. Chem. 2016, 59, 10661–10675.
- 70 Bartholomäus, R.; Dommershausen, F.; Thiele, M.; Karanjule, N. S.; Harms, K.; Koert, U. Total Synthesis of the Postulated Structure of Fulicineroside. Chem. Eur. J. 2013, 19, 7423–7436.
- 71 Park, S.; Kim, S.-H.; Jeong, J.-H.; Shin, D. Total Synthesis of Giffonin H by Fluoride-Catalyzed Macrocyclization. Org. Chem. Front. 2019, 6, 704–708.
- 72 Anugu, R. R.; Mainkar, P. S.; Sridhar, B.; Chandrasekhar, S. The Ireland-Claisen Rearrangement Strategy towards the Synthesis of the Schizophrenia Drug, (+)-Asenapine. Org. Biomol.Chem. 2016, 14, 1332–1337.
- 73 Lee, W.-G.; Gallardo-Macias, R.; Frey, K. M.; Spasov, K. A.; Bollini, M.; Anderson, K. S.; Jorgensen, W. L. J. Am. Chem. Soc. 2013, 135, 16705–16713.
- 74Boyd, S.; Brookfield, J. L.; Critchlow, S. E.; Cumming, I. A.; Curtis, N. J.; Debreczeni, J.; Degorce, S. L.; Donald, C.; Evans, N. J.; Groombridge, S.; Hopcroft, P.; Jones, N. P.; Kettle, J. G.; Lamont, S.; Lewis, H. J.; MacFaull, P.; McLoughlin, S. B.; Rigoreau, L. J. M.; Smith, J. M.; St-Gallay, S.; Stock, J. K.; Turnbull, A. P.; Wheatley, E. R.; Winter, J.; Wingfield, J. Structure-Based Design of Potent and Selective Inhibitors of the Metabolic Kinase PFKFB3. J. Med. Chem. 2015, 58, 3611–3625.
- 75 Palmer, W. S.; Poncet-Montange, G.; Liu, G.; Petrocchi, A.; Reyna, N.; Subramanina, G.; Theroff, J.; Yau, A.; Kost-Alimova, M.; Bardenhagen, J. P.; Leo, E.; Shepard, H. E.; Tieu, T. N.; Shi, X.; Zhan, Y.; Zhao, S.; Barton, M. C.; Draetta, G.; Toniatti, C.; Jones, P.; Do, M. G.; Andersen, J. N. Structure-Guided Design of IACS-9571, a Selective High-Affinity Dual TRIM24-BRPF1 Bromodomain Inhibitor. J. Med. Chem. 2016, 59, 1440–1454.
- 76 Rutaganira, F. U.; Barks, J.; Dhason, M. S.; Wang, Q.; Lopez, M. S.; Long, S.; Radke, J. B.; Jones, N. G.; Maddirala, A. R.; Janetka, J. W.; Bakkouri, M. E.; Hui, R.; Shokat, K. M.; Sibley, L. D. Inhibition of Calcium Dependent Protein Kianse 1 (CDPK1) by Pyrazolopyrimidine Analogs Decreases Establishment and Reoccurrence of Central Nervous System Disease by Toxoplasma gondii. J. Med. Chem. 2017, 60, 9976–9989.
- 77 Song, L.; Merceron, R.; Gracia, B.; Quintana, A. L.; Risseeuw, M. D. P.; Hulpia, F.; Cos, P.; Aínsa, J. A.; Munier-Lehmann, H.; Savvides, S. N.; Calenbergh, S. V. Structure Guilded Lead Generation toward Nonchiral M. tuberculosis Thymidylate Kinase Inhibitors. J. Med. Chem. 2018, 61, 2753–2775.
- 78 Le, T. G.; Kundu, A.; Ghoshal, A.; Nguyen, N. H.; Preston, S.; Jiao, Y.; Ruan, B.; Xue, L.; Huang, F.; Keiser, J.; Hofmann, A.; Chang, B. C. H.; Garcia-Bustos, J.; Wells, T. N. C.; Palmer, M. J.; Jabbar, A.; Gasser, R. B.; Baell, J. B. Structure-Activity Relationship Studies of Tolfenpyrad Reveal Subnanomolar Inhibitors of Haemonchus contortus Development. J. Med. Chem. 2019, 62, 1036–1053.
- 79 de Lange, B.; Hyett, D. J.; Maas, P. J.; Mink, D.; van Assema, F. B. J.; Sereinig, N.; de Vries, A. H. M.; de Vries, J. G. Asymmetric Synthesis of (S)-2-Indolinecarboxylic Acid by Combining Biocatalysis and Homogeneous Catalysis. ChemCatChem 2011, 3, 289–292.
- 80(a) Flick, A. C.; Ding, H. X.; Leverett, C. A.; Fink, S. J.; O'Donnell, C. J. Synthetic Approaches to New Drugs Approved During 2016. J. Med. Chem. 2018, 61, 7004–7031; (b) Zeller, J. R.; Venkatraman, S.; Brot, E. C. A.; Iyer, S.; Hall, M. LFA-1 Inhibitor and Polymorph Thereof. WO2014/018748.
- 81 Ribecai, A.; Bacchi, S.; Delpogetto, M.; Guelfi, S.; Manzo, A. M.; Perboni, A.; Stabile, P.; Westerduin, P.; Hourdin, M.; Rossi, S.; Provera, S.; Turco, L. Identification of a Manufacturing Route of Novel CRF-1 Antagonists Containing a 2,3-Dihydro-1H-pyrrolo[2,3-b]pyridine Moiety. Org. Process Res. Dev. 2010, 14, 895–901.
- 82 Enache, L. A.; Kennedy, I.; Sullins, D. W.; Chen, W.; Ristic, D.; Stahl, G. L.; Dzekhtser, S.; Erickson, R. A.; Yan, C.; Muellner, F. W.; Krohn, M. D.; Winger, J.; Sandanayaka, V.; Singh, J.; Zembower, D. E.; Kiselyov, A. S. Development of a Scalable Synthetic Process for DG-051B, A First-in-Class Inhibitor of LTA4H. Org. Process Res. Dev. 2009, 13, 1177–1184.
- 83 Pulman, D. A. Deltamethrin: The Cream of the Crop. J. Agric. Food Chem. 2011, 59, 2770–2772.
- 84 Schareina, T.; Zapf, A.; Cotté, A.; Müller, N.; Beller, M. A Practical and Improved Copper-Catalyzed Synthesis of the Central Intermediate of Diafenthiuron and Related Products. Org. Process Res. Dev. 2008, 12, 537–539.
- 85 Nakamura, K.; Furumi, S.; Takeuchi, M.; Shibuya, T.; Tanaka, K. Enantioselective Synthesis and Enhanced Circularly Polarized Luminescence of S-Shaped Double Azahelicenes. J. Am. Chem. Soc. 2014, 136, 5555–5558.
- 86 Xu, H.; Yang, D.; Liu, F.; Fu, M.; Bo, S.; Liu, X.; Cao, Y. Nonliner Optical Chromophores Based on Dewar's Rules: Enhancement of Electro- Optic Activity by Introducing Heteroatoms into the Donor or Bridge. Phys. Chem. Chem. Phys. 2015, 17, 29679–29688.
- 87 Zhang, X.-Q.; Xie, Y.-M.; Zheng, Y.; Liang, F.; Wang, B.; Fan, J.; Liao, L.-S. Highly Phosphorescent Platinum(II) Complexes Based on Rigid Unsymmetric Tetradentate Ligands. Org. Electron. 2016, 32, 120–125.
- 88 Ruff, Y.; Berst, F. Efficient Copper-Catalyzed Amination of DNA-Conjugated Aryl Iodides under Mild Aqueous Conditions. Med. Chem. Commun. 2018, 9, 1188–1193.
- 89(a) Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd Metal Catalysts for Cross-Coupling and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295; (b) Johansson Seechurn, C. C. C.; Kitching, M. O.; Colacot, T. J.; Snieckus, V. Palladium-Catalyzed Cross-Couping: A Histroical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085.
- 90(a) Zhou, F.; Cai, Q. Recent Advances in Copper-Catalyzed Asymmetric Coupling Reactions. Beilstein J. Org. Chem. 2015, 11, 2600–2615; (b) Zhou, F.; Liu, J.; Cai, Q. Transition Metal Catalyzed Asymmetric Aryl Carbon-Heteroatom Bond Coupling Reactions. Synlett 2016, 27, 664–675.
- 91(a) Fu, W.; Tang, W. Chiral Monophosporus Ligands for Asymmetric Catalytic Reactions. ACS Catal. 2016, 6, 4814–4858; (b) Yeh, V.; Szabo, W. A. Asymmetric Cross-Coupling Reactions. In Applications of Transition Metal Catalysis in Drug Discovery and Development: An Industrial Perspective, Eds.: Crawley, M. L.; Trost, B. M., John Wiley & Sons, Inc., New York, NY, 2012, pp. 165–213.




