Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture
- Page: 245
- First Published: 01 January 2025

Chiral 1,3-substituted fragments are ubiquitous in pharmaceutical molecules and natural products, prompting the development of numerous methods to access these structures. Herein, we developed a nickel-catalyzed enantioselective migratory arylboration reaction of boron-containing alkenes using a chiral 1,2-diamine ligand, yielding a range of chiral 1,3-bis(boronates) with high enantioselectivity. The protocol is characterized by its simple operation, good substrate tolerance, and high application value. More details are discussed in the article by Yin et al. on pages 261—267.
Inside Cover Picture
Inside Cover Picture
- Page: 246
- First Published: 01 January 2025

gem-Difluoroalkenes are highly regarded for their use as fluorinated synthetic building blocks and are also recognized for their numerous applications in the pharmaceuticals. A practical zinc-mediated approach for the synthesis of 3-gem-difluorovinyl lactams through a sequential reductive hydrodehalogenation has been disclosed. Additionally, such a strategy could also enable the generation of the high value-added vicinal dideuterated gem-difluoroalkenes with excellent deuterium (D) incorporation at both α- and β-positions with the presence of inexpensive deuterium oxide. More details are discussed in the article by Hu et al. on pages 255—260.
Contents
Concise Report
Enabling Access to 3-gem-Difluorovinyl Lactams via Zn-Mediated Sequential Single Electron Reductive Hydrodehalogenation
- Pages: 255-260
- First Published: 17 October 2024
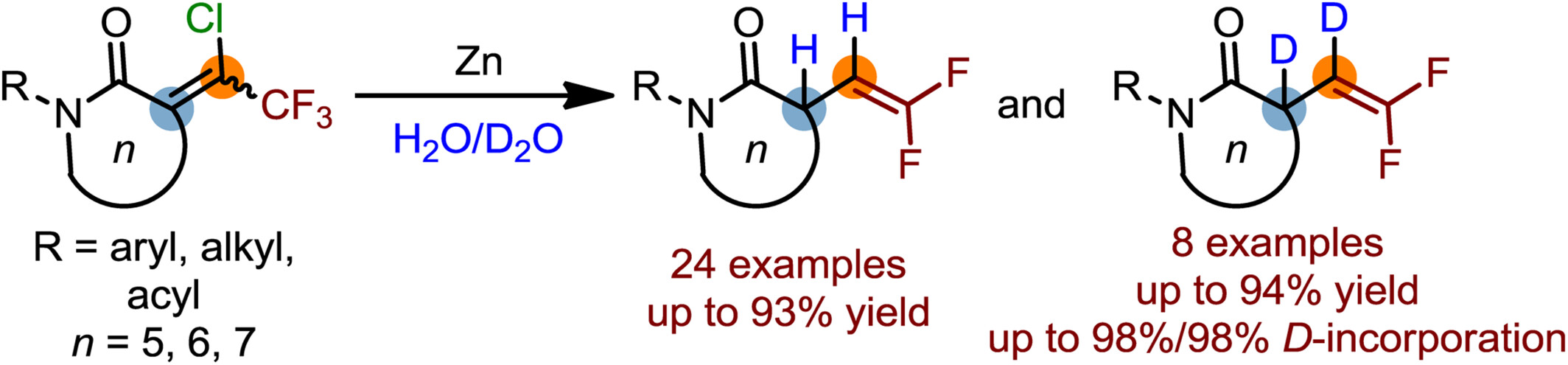
We report a practical method for synthesis of 3-gem-difluorovinyl lactams and their dideuterated derivatives with excellent deuterium (D) incorporation. This zinc-mediated sequential reductive hydrodehalogenation proceeds via a radical process. The in-situ generated zinc cation complex plays a key role in facilitating the transformation due to its strong Lewis acidity.
Modular Access to Chiral 1,3-Substituted Fragments via Nickel-Catalyzed Arylboration Reaction
- Pages: 261-267
- First Published: 17 October 2024
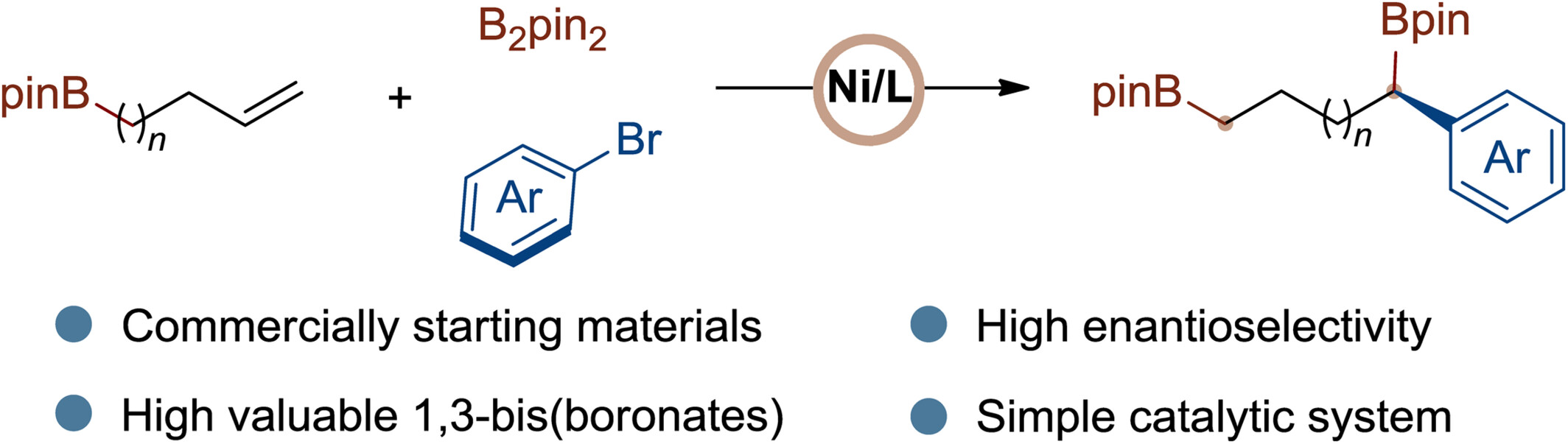
The chiral 1,3-substituted skeleton is a fundamental structure present in organic molecules, and thousands of methods have been developed for this purpose. Herein, we report a nickel-catalyzed migratory carboboration of boron-containing alkenes for the preparation of chiral 1,3-diboron compounds, which serve as versatile precursors to 1,3-substituted skeletons. This method is featured by its simple operation, good substrate tolerance, and high application value.
Penijanacoranes A—F, Acorane-Type Sesquiterpenes from a Deep Sea-Derived Fungus Penicillium janthinellum SH0301
- Pages: 268-274
- First Published: 17 October 2024

Six new acorane-type sesquiterpenes, penijanacoranes A—F (1—6) were isolated from the YES medium of the deep-sea-derived fungus P. janthinellum SH0301. Penijanacorane A (1) was identified as a rare acorane-type sesquiterpene lactone with a novel 6/5/6 tricyclic system, while penijanacoranes E—F (5—6) were elucidated as undescribed examples of nor-acorane sesquiterpenes at C-1.
Dual Photoredox and Titanium Catalyzed Regioselective Allenylation of Aldehydes via Reductive Radical-Polar Crossover
- Pages: 275-280
- First Published: 25 October 2024
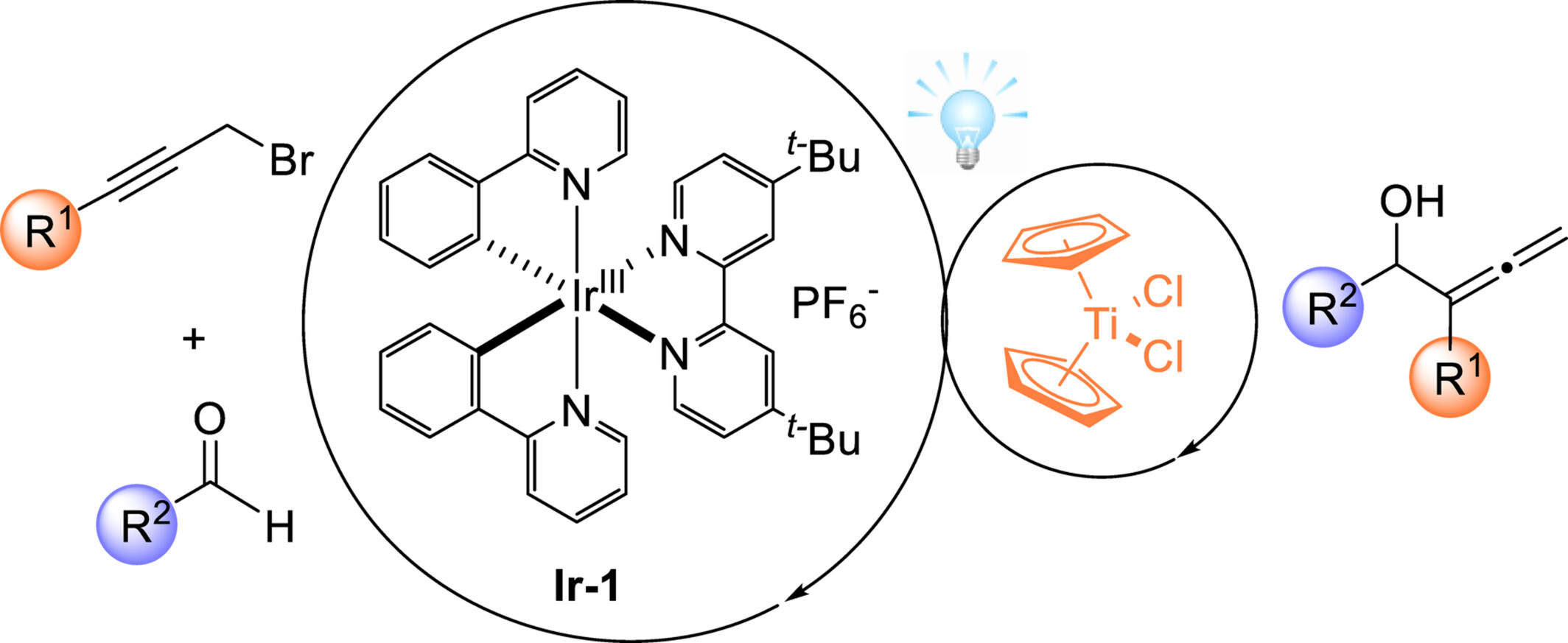
We developed the Ti-catalyzed regioselective reductive coupling of readily available aldehydes and racemic propargyl bromides to rapidly access a wide range of α-allenols. This method proceeds efficiently in a reductive radical-polar crossover manner featuring mild conditions, excellent regioselectivity control, broad substrate scope, and eco-friendliness. Preliminary mechanistic studies support the radical-involved catalytic cycle. DFT calculations demonstrate that the regioselectivity is determined by the Zimmerman-Traxler-type transition states.
Mechanism of TMB Discoloration Catalyzed by Layered CoNi@CN Nanozymes: Application Based on Smart Phone for Resorcinol Detection
- Pages: 281-291
- First Published: 25 October 2024
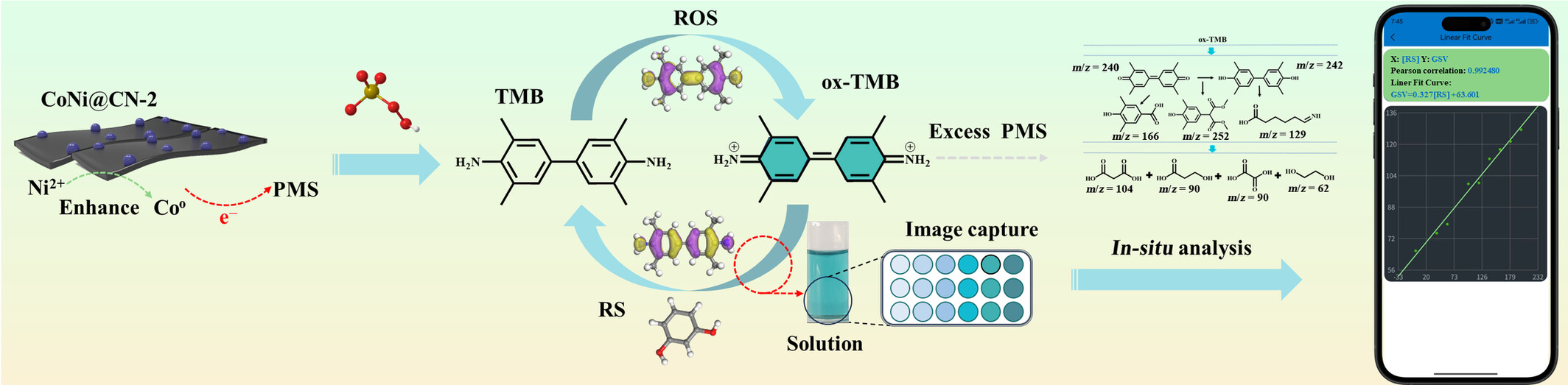
The oxidation of Ni0 on the surface of CoNi@CN-2 nanoenzymes generates Ni2+, which significantly enhances the electron-donating ability of Co0. When excess PMS is introduced, it results in reactive oxygen species (ROS) such as SO4•−, •O2−, •OH and 1O2 attacking specific sites in ox-TMB. Additionally, a new smartphone-integrated autonomous detection software has been developed to improve the accuracy and sensitivity of detecting RS in environmental water.
Electrochemical Synthesis of Alkenylsulfonates from Alkynes, NaHSO3 and Alcohols
- Pages: 292-296
- First Published: 07 November 2024
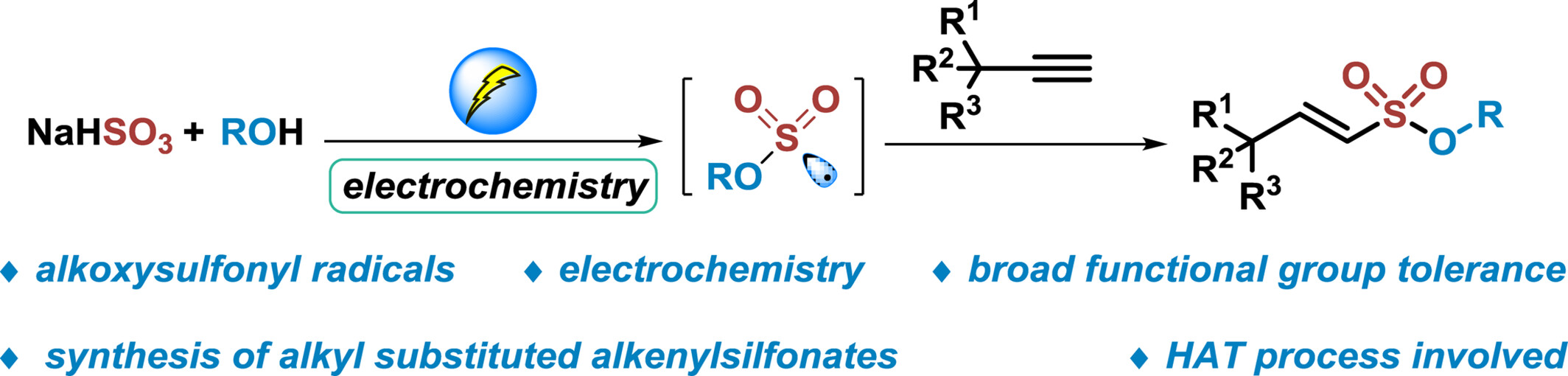
An electrochemical reaction involving alkynes, NaHSO3, and alcohols has been developed. This method yields functionalized alkenylsulfonates in good yields with broad functional group tolerance. Mechanism studies indicate that anodic oxidation of inorganic sulfite, radical insertion process, and HAT process are involved in this transformation.
Enhancing Oxygen Reduction Reaction Electrocatalytic Performance of Nickel-Nitrogen-Carbon Catalysts through Coordination Environment Engineering
- Pages: 297-307
- First Published: 08 November 2024
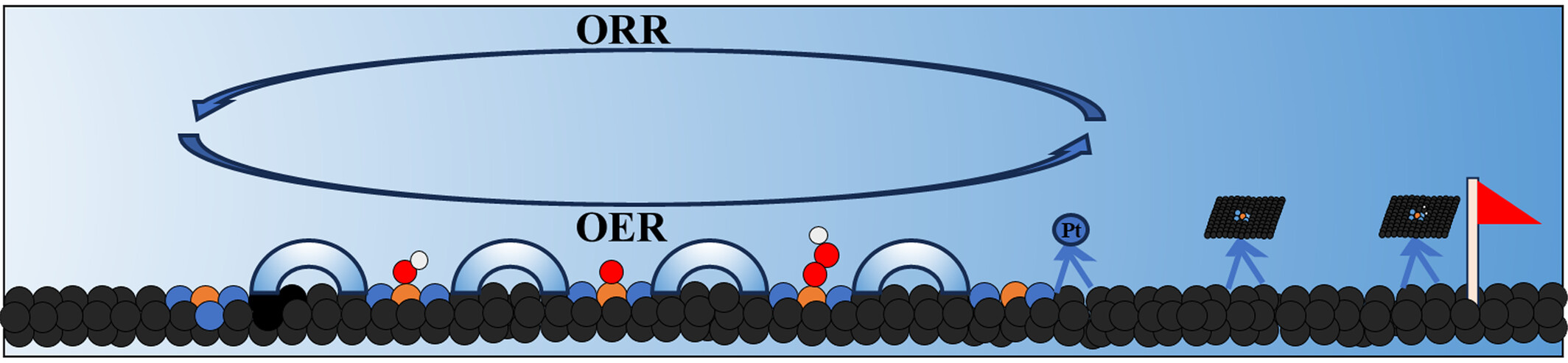
The design and development of efficient bifunctional electrocatalysts for fuel cells and rechargeable metal-air batteries have become increasingly urgent. In this study, our investigation reveals that NiN3H2 consistently exhibits optimal ORR activity across a wide pH range, regardless of the source of proton-electron pair (solvent or catalyst surface).
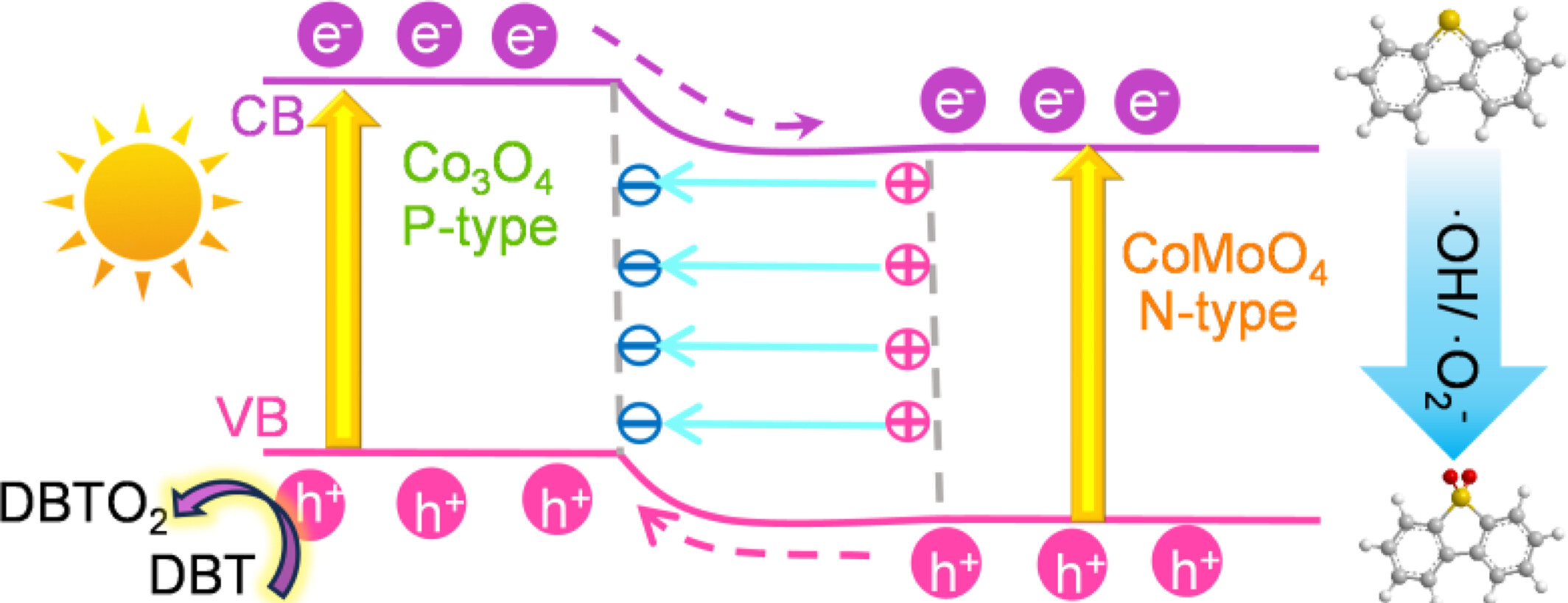
Two-Dimensional Janus p-n Heterojunction of Co3O4 and CoMoO4 for Boosting Photocatalytic Oxidative Desulfurization
- Pages: 308-314
- First Published: 20 November 2024
Recent Advances
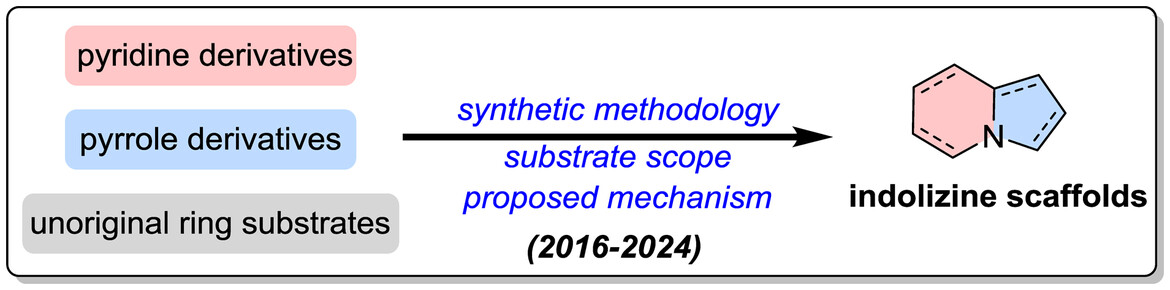
Recent Advances in the Development of Indolizine Scaffolds: Synthetic Methodology and Mechanistic Insights
- Pages: 315-348
- First Published: 20 November 2024
Emerging Topic
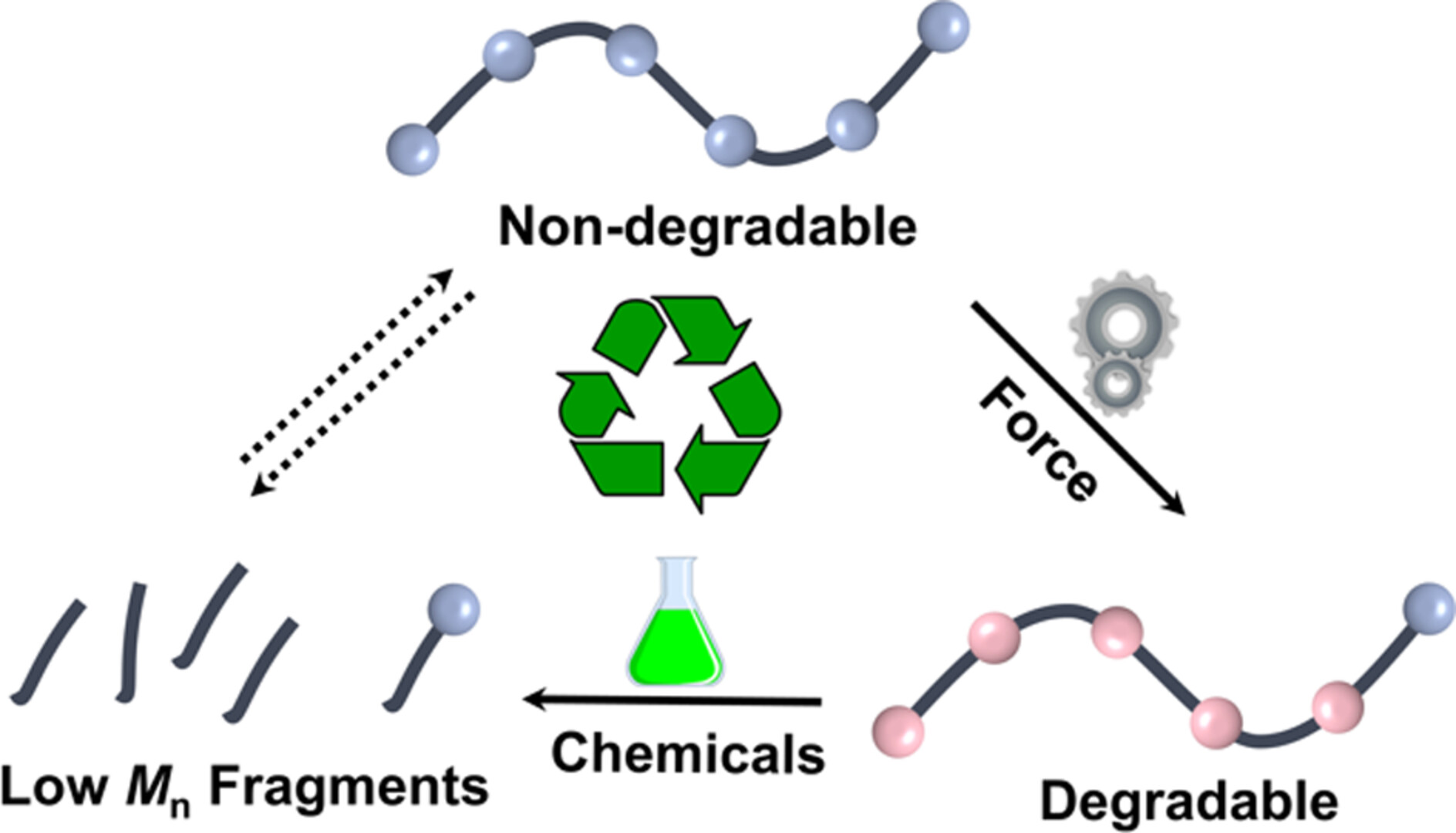
Polymer Degradation via a Mechanochemical Gating Strategy
- Pages: 349-354
- First Published: 20 November 2024
Back Cover
Back Cover
- Page: 360
- First Published: 01 January 2025

The cover picture displays six new acorane-type sesquiterpenes penijanacoranes A—F isolated from a Mariana Trench-derived fungus Penicillium janthinellum SH0301. Penijanacorane A was a rare acorane-type sesquiterpene lactone featuring a novel 6/5/6 tricyclic system, and penijanacoranes E and F represented undescribed examples of nor-acorane sesquiterpenes at C-1. Penijanacorane C exhibited significant inhibitory activity against LPS-induced NO production in Raw264.7 macrophages. More details are discussed in the article by Wang et al. on pages 268—274.