Penijanacoranes A—F, Acorane-Type Sesquiterpenes from a Deep Sea-Derived Fungus Penicillium janthinellum SH0301
Lu-Jia Yang
MOE Key Laboratory of Marine Drugs and Key Laboratory of Evolution and Marine Biodiversity, School of Medicine and Pharmacy; Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao, Shandong, 266237 China
Search for more papers by this authorLing Lv
MOE Key Laboratory of Marine Drugs and Key Laboratory of Evolution and Marine Biodiversity, School of Medicine and Pharmacy; Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao, Shandong, 266237 China
Search for more papers by this authorZhuang Han
Institute of Deep-sea Science and Engineering, Chinese Academy of Sciences, Sanya, Hainan, 572000 China
Search for more papers by this authorYu-Cheng Gu
Syngenta Jealott's Hill International Research Centre, Bracknell, Berkshire, RG42 6EY United Kingdom
Search for more papers by this authorXin Li
MOE Key Laboratory of Marine Drugs and Key Laboratory of Evolution and Marine Biodiversity, School of Medicine and Pharmacy; Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao, Shandong, 266237 China
Search for more papers by this authorChang-Lun Shao
MOE Key Laboratory of Marine Drugs and Key Laboratory of Evolution and Marine Biodiversity, School of Medicine and Pharmacy; Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao, Shandong, 266237 China
Search for more papers by this authorCorresponding Author
Zhi-Qing Liu
MOE Key Laboratory of Marine Drugs and Key Laboratory of Evolution and Marine Biodiversity, School of Medicine and Pharmacy; Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao, Shandong, 266237 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chang-Yun Wang
MOE Key Laboratory of Marine Drugs and Key Laboratory of Evolution and Marine Biodiversity, School of Medicine and Pharmacy; Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao, Shandong, 266237 China
E-mail: [email protected]; [email protected]Search for more papers by this authorLu-Jia Yang
MOE Key Laboratory of Marine Drugs and Key Laboratory of Evolution and Marine Biodiversity, School of Medicine and Pharmacy; Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao, Shandong, 266237 China
Search for more papers by this authorLing Lv
MOE Key Laboratory of Marine Drugs and Key Laboratory of Evolution and Marine Biodiversity, School of Medicine and Pharmacy; Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao, Shandong, 266237 China
Search for more papers by this authorZhuang Han
Institute of Deep-sea Science and Engineering, Chinese Academy of Sciences, Sanya, Hainan, 572000 China
Search for more papers by this authorYu-Cheng Gu
Syngenta Jealott's Hill International Research Centre, Bracknell, Berkshire, RG42 6EY United Kingdom
Search for more papers by this authorXin Li
MOE Key Laboratory of Marine Drugs and Key Laboratory of Evolution and Marine Biodiversity, School of Medicine and Pharmacy; Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao, Shandong, 266237 China
Search for more papers by this authorChang-Lun Shao
MOE Key Laboratory of Marine Drugs and Key Laboratory of Evolution and Marine Biodiversity, School of Medicine and Pharmacy; Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao, Shandong, 266237 China
Search for more papers by this authorCorresponding Author
Zhi-Qing Liu
MOE Key Laboratory of Marine Drugs and Key Laboratory of Evolution and Marine Biodiversity, School of Medicine and Pharmacy; Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao, Shandong, 266237 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chang-Yun Wang
MOE Key Laboratory of Marine Drugs and Key Laboratory of Evolution and Marine Biodiversity, School of Medicine and Pharmacy; Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao, Shandong, 266237 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
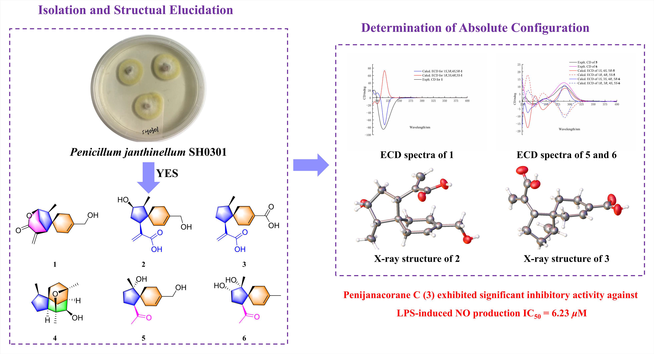
Six new acorane-type sesquiterpenes, named penijanacoranes A—F (1—6), as well as one known eudesmane sesquiterpenoid 1α,6β,11-eudesm-triol (7) have been isolated from a deep-sea-derived fungus Penicillium janthinellum SH0301. Their structures and absolute configurations were established by the comprehensive spectroscopic analysis, TDDFT-ECD calculations, and X-ray diffraction. Penijanacorane A (1) was identified as a rare acorane-type sesquiterpene lactone featuring a novel 6/5/6 tricyclic system, while penijanacoranes E and F (5 and 6) represented undescribed examples of nor-acorane sesquiterpenes at C-1. Penijanacorane C (3) exhibited significant inhibitory activity against LPS-induced NO production in Raw264.7 macrophages with an IC50 value of 6.23 μM, which was more potent than that of positive control dexamethasone (IC50 = 11.49 μM). This study expanded the chemical diversity of acorane-type sesquiterpenoids and revealed that compound 3 was a potential molecule for anti-inflammatory agents.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400836-sup-0001-supinfo.pdfPDF document, 4.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Jiang, M.; Wu, Z.; Guo, H.; Liu, L.; Chen, S. A Review of Terpenes from Marine-Derived Fungi: 2015-2019. Mar. Drugs 2020, 18, 321.
- 2 Xiong, J.; Wang, L. J.; Qian, J.; Wang, P. P.; Wang, X. J.; Ma, G. L.; Zeng, H.; Li, J.; Hu, J. F. Structurally Diverse Sesquiterpenoids from the Endangered Ornamental Plant Michelia shiluensis. J. Nat. Prod. 2018, 81, 2195–2204.
- 3 Li, H.; Huang, H.; Shao, C.; Huang, H.; Jiang, J.; Zhu, X.; Liu, Y.; Liu, L.; Lu, Y.; Li, M.; Lin, Y.; She, Z. Cytotoxic Norsesquiterpene Peroxides from the Endophytic Fungus Talaromyces flavus Isolated from the Mangrove Plant Sonneratia apetala. J. Nat. Prod. 2011, 74, 1230–1235.
- 4 Mcclure, R. J.; Schorno, K. S.; Bertrand, J. A.; Zalko, L. H. The Structure and Stereochemistry of a New Sesquiterpene of the Acorane Type. Chem. Commun. 1968, 11135–1136.
- 5 Bao, A.; Li, Q.; Zhang, X.; Zheng, M.; Ding, N.; Chen, C.; Zhang, Y.; Zhu, H. Proliferacorins A-M: Thirteen Undescribed Acorane Sesquiterpenoids Isolated from the Fungus Fusarium proliferatum. Phytochemistry 2024, 221, 114065.
- 6 Yan, H.; Xu, L. L.; Zheng, X. F.; Zou, X. F.; Xiao, L. G.; Zhou, Y. S.; He, L.; Liu, H. Y. Sesquiterpenes from Chloranthus holostegius with Anti-inflammatory Activities. Fitoterapia 2024, 172, 105766.
- 7 Zhou, M. H.; Luo, X. C.; Zhao, H. M.; Lu, J. R.; Dai, Y.; Yu, Y.; Zhang, L.; Lin, H. W.; Yang, F. New Spiro-Sesquiterpenoids from the Marine Sponge Myrmekioderma sp. Chem. Biodivers. 2022, 19, e202200455.
- 8 Zhang, W.; Meng, Q.; Wu, J.; Cheng, W.; Liu, D.; Huang, J.; Fan, A.; Xu, J.; Lin, W. Acorane Sesquiterpenes from the Deep-Sea Derived Penicillium bilaiae Fungus with Anti-neuroinflammatory Effects. Front. Chem. 2022, 10, 1036212.
- 9 Yong, J. Y.; Li, M.; Li, W. R.; Gao, R. M.; Su, G. Z.; Wang, H. Q.; Yang, J.; Li, L.; Li, Y. H.; Scott, P.; Wang, R. B.; Wang, X. J.; Ma, S. G. seco-Sesquiterpenes and Acorane-type Sesquiterpenes with Antiviral Activity from the Twigs and Leaves of Illicium henryi Diels. Bioorg. Chem. 2023, 131, 106324.
- 10 Sandargo, B.; Michehl, M.; Praditya, D.; Steinmann, E.; Stadler, M.; Surup, F. Antiviral Meroterpenoid Rhodatin and Sesquiterpenoids Rhodocoranes A-E from the Wrinkled Peach Mushroom, Rhodotus palmatus. Org. Lett. 2019, 21, 3286–3289.
- 11 Li, G. H.; Yang, Z. S.; Zhao, P. J.; Zheng, X.; Luo, S. L.; Sun, R.; Niu, X. M.; Zhang, K. Q. Three New Acorane Sesquiterpenes from Trichoderma sp. YMF1.02647. Phytochem. Lett. 2011, 4, 86–88.
- 12 Liu, M. Y.; Zhang, X. W.; Li, G. Q. Structural and Biological Insights into the Hot-Spot Marine Natural Products Reported from 2012 to 2021. Chin. J. Chem. 2022, 40, 1867–89
- 13 Ma, H. G.; Liu, Q.; Zhu, G. L.; Liu, H. S.; Zhu, W. M. Marine Natural Products Sourced from Marine-Derived Penicillium fungi. J. Asian Nat. Prod. Res. 2016, 18, 92–115.
- 14 Liu, S.; Su, M.; Song, S. J.; Jung, J. H. Marine-Derived Penicillium Species as Producers of Cytotoxic Metabolites. Mar. Drugs 2017, 15, 329.
- 15 Zhang, P.; Wei, Q.; Yuan, X.; Xu, K. Newly Reported Alkaloids Produced by Marine-Derived Penicillium Species (covering 2014-2018). Bioorg. Chem. 2020, 99, 103840.
- 16 Chen, Q.; Gao, J.; Jamieson, C.; Liu, J.; Ohashi, M.; Bai, J.; Yan, D.; Liu, B.; Che, Y.; Wang, Y.; Houk, K. N.; Hu, Y. Enzymatic Intermolecular Hetero-Diels-Alder Reaction in the Biosynthesis of Tropolonic Sesquiterpenes. J. Am. Chem. Soc. 2019, 141, 14052–14056.
- 17 Guo, X. C.; Xu, L. L.; Yang, R. Y.; Yang, M. Y.; Hu, L. D.; Zhu, H. J.; Cao, F. Anti-Vibrio Indole-Diterpenoids and C-25 Epimeric Steroids from the Marine-Derived Fungus Penicillium janthinellum. Front. Chem. 2019, 7, 80.
- 18 Chen, M.; Shen, N. X.; Chen, Z. Q.; Zhang, F. M.; Chen, Y. Penicilones A-D, Anti-MRSA Azaphilones from the Marine-Derived Fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 2017, 80, 1081–1086.
- 19 Chen, M.; Zheng, Y. Y.; Chen, Z. Q.; Shen, N. X.; Shen, L.; Zhang, F. M.; Zhou, X. J.; Wang, C. Y. NaBr-Induced Production of Brominated Azaphilones and Related Tricyclic Polyketides by the Marine-Derived Fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 2019, 82, 368–374.
- 20 Zhu, M.; Yang, Z.; Wang, H.; Gan, Q.; Zhang, G.; Che, Q.; Zhu, T.; Gu, Q.; Han, B.; Li, D. Penispirozines A-H, Three Classes of Dioxopiperazine Alkaloids with Spirocyclic Skeletons Isolated from the Mangrove-Derived Penicillium janthinellum. J. Nat. Prod. 2020, 83, 2647–2654.
- 21 Zhu, M.; Zhang, X.; Feng, H.; Dai, J.; Li, J.; Che, Q.; Gu, Q.; Zhu, T.; Li, D. Penicisulfuranols A-F, Alkaloids from the Mangrove Endophytic Fungus Penicillium janthinellum HDN13-309. J. Nat. Prod. 2017, 80, 71–75.
- 22 Li, R.; Zhou, Y.; Zhang, X.; Yang, L.; Liu, J.; Wightman, S. M.; Lv, L.; Liu, Z.; Wang, C. Y.; Zhao, C. Identification of Marine Natural Product Pretrichodermamide B as a STAT3 Inhibitor for Efficient Anticancer Therapy. Mar. Life. Sci. Technol. 2023, 5, 94–101.
- 23 Hu, Z. F.; Qin, L. L.; Ding, W. J.; Liu, Y.; Ma, Z. J. New Analogues of Brefeldin A from Sediment-Derived Fungus Penicillium sp. DT-F29. Nat. Prod. Res. 2016, 30, 2311–2315.
- 24 Cheng, X.; Yu, L.; Wang, Q.; Ding, W.; Chen, Z.; Ma, Z. New Brefeldins and Penialidins from Marine Fungus Penicillium janthinellum DT-F29. Nat. Prod. Res. 2018, 32, 282–286.
- 25
Liu, L.; Zheng, Y. Y.; Shao, C. L.; Wang, C. Y. Metabolites from Marine Invertebrates and Their Symbiotic Microorganisms: Molecular Diversity Discovery, Mining, and Application. Mar. Life. Sci. Technol. 2019, 1, 60–94.
10.1007/s42995-019-00021-2 Google Scholar
- 26 Yang, Z.; Yang, Y. B.; Yang, X. Q.; Zhang, Y.; Zhao, L. X.; Xu, L. H.; Ding, Z. T. Sesquiterpenes from the Secondary Metabolites of Streptomyces sp (YIM 56130). Chem. Pharm. Bull. 2011, 59, 1430–1433.
- 27 Tamiya, J.; Sorensen, E. J. A Spontaneous Bicyclization Facilitates a Synthesis of (−)-Hispidospermidin. Tetrahedron 2003, 59, 6921–6932.
- 28 Citron, C. A.; Riclea, R.; Brock, N. L.; Dickschat, J. S. Biosynthesis of Acorane Sesquiterpenes by Trichoderma. RSC Adv. 2011, 1, 290–297.
- 29 Guo, R.; Ren, Q.; Tang, Y. X.; Zhao, F.; Lin, B.; Huang, X. X.; Song, S. J. Sesquiterpenoids from the Roots of Daphne genkwa Siebold et Zucc. with Potential Anti-Inflammatory Activity. Phytochemistry 2020, 174, 112348.
- 30 Lv, L.; Maimaitiming, M.; Huang, Y.; Yang, J.; Chen, S.; Sun, Y.; Zhang, X.; Li, X.; Xue, C.; Wang, P.; Wang, C. Y.; Liu, Z. Discovery of Quinazolin-4(3H)-one Derivatives as Novel AChE Inhibitors with Anti-Inflammatory Activities. Eur. J. Med. Chem. 2023, 254, 115346.
- 31 Ferreira, P. C. P.; Peixoto, M. L. P.; Silva, M. A. V.; Golgher, R. R. Assay of Human Interferon in Vero Cells by Several Methods. J. Clin. Microbiol. 1979, 9, 471–475.
- 32 Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J. T.; Bokesch, H.; Kenney, S.; Boyd, M. R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer. Inst. 1990, 82, 1107–1112.




