Electrochemical Synthesis of Alkenylsulfonates from Alkynes, NaHSO3 and Alcohols
Xiaoman Wang
State Key Laboratory of Materials-Oriented Chemical Engineering, School of Pharmaceutical Sciences, Nanjing Tech University, Nanjing, Jiangsu, 210009 China
Search for more papers by this authorQianqian Chen
School of Pharmaceutical and Chemical Engineering & Institute for Advanced Studies, Taizhou University, 1139 Shifu Avenue, Taizhou, Zhejiang, 318000 China
Search for more papers by this authorJinhang Zhou
School of Pharmaceutical and Chemical Engineering & Institute for Advanced Studies, Taizhou University, 1139 Shifu Avenue, Taizhou, Zhejiang, 318000 China
Search for more papers by this authorCorresponding Author
Yi Hu
State Key Laboratory of Materials-Oriented Chemical Engineering, School of Pharmaceutical Sciences, Nanjing Tech University, Nanjing, Jiangsu, 210009 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Shengqing Ye
School of Pharmaceutical and Chemical Engineering & Institute for Advanced Studies, Taizhou University, 1139 Shifu Avenue, Taizhou, Zhejiang, 318000 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jie Wu
School of Pharmaceutical and Chemical Engineering & Institute for Advanced Studies, Taizhou University, 1139 Shifu Avenue, Taizhou, Zhejiang, 318000 China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorXiaoman Wang
State Key Laboratory of Materials-Oriented Chemical Engineering, School of Pharmaceutical Sciences, Nanjing Tech University, Nanjing, Jiangsu, 210009 China
Search for more papers by this authorQianqian Chen
School of Pharmaceutical and Chemical Engineering & Institute for Advanced Studies, Taizhou University, 1139 Shifu Avenue, Taizhou, Zhejiang, 318000 China
Search for more papers by this authorJinhang Zhou
School of Pharmaceutical and Chemical Engineering & Institute for Advanced Studies, Taizhou University, 1139 Shifu Avenue, Taizhou, Zhejiang, 318000 China
Search for more papers by this authorCorresponding Author
Yi Hu
State Key Laboratory of Materials-Oriented Chemical Engineering, School of Pharmaceutical Sciences, Nanjing Tech University, Nanjing, Jiangsu, 210009 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Shengqing Ye
School of Pharmaceutical and Chemical Engineering & Institute for Advanced Studies, Taizhou University, 1139 Shifu Avenue, Taizhou, Zhejiang, 318000 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jie Wu
School of Pharmaceutical and Chemical Engineering & Institute for Advanced Studies, Taizhou University, 1139 Shifu Avenue, Taizhou, Zhejiang, 318000 China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
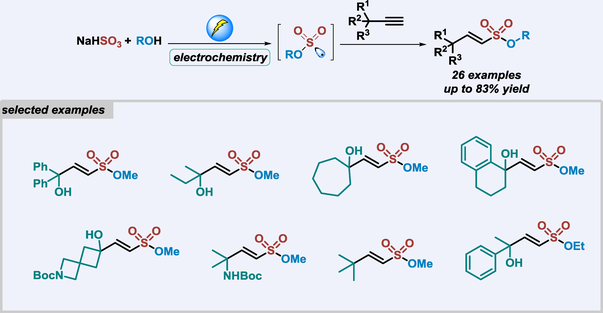
Alkenylsulfonates are commonly encountered in medicinal chemistry, polymer chemistry, and organic synthesis. In this research, an electrochemical reaction involving alkynes, NaHSO3, and alcohols has been developed. This method yields functionalized alkenylsulfonates in good yields with broad functional group tolerance. Mechanism studies indicate that anodic oxidation of inorganic sulfite, radical insertion process, and HAT process are involved in this transformation.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400962-sup-0001-supinfo.pdfPDF document, 1.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Roush, W. R.; Gwaltney, S. L.; Cheng, J.; Scheidt, K. A.; McKerrow, J. H.; Hansell, E. Vinyl Sulfonate Esters and Vinyl Sulfonamides: Potent, Irreversible Inhibitors of Cysteine Proteases. J. Am. Chem. Soc. 1998, 120, 10994–10995; (b) Liu, S.; Zhou, B.; Yang, H.; He, Y.; Jiang, Z.-X.; Kumar, S.; Wu, L.; Zhang, Z.-Y. Aryl Vinyl Sulfonates and Sulfones as Active Site-Directed and Mechanism-Based Probes for Protein Tyrosine Phosphatases. J. Am. Chem. Soc. 2008, 130, 8251–8260; (c) Gushwa, N. N.; Kang, S.; Chen, J.; Taunton, J. Selective Targeting of Distinct Active Site Nucleophiles by Irreversible Src-Family Kinase Inhibitors. J. Am. Chem. Soc. 2012, 134, 20214–20217; (d) Yang, F.; Xie, F.; Zhang, Y.; Xia, Y.; Liu, W.; Jiang, F.; Lam, C.; Qiao, Y.; Xie, D.; Li, J.; Fu, L. Y-shaped bis-arylethenesulfonic acid esters: Potential potent and membrane permeable protein tyrosine phosphatase 1B inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 2166–2170; (e) Choi, J. W.; Shin, S. J.; Kim, H. J.; Park, J.-H.; Kim, H. J.; Lee, E. H.; Pae, A. N.; Bahn, Y. S.; Park, K. D. Antioxidant, Anti-inflammatory, and Neuroprotective Effects of Novel Vinyl Sulfonate Compounds as Nrf2 Activator. ACS Med. Chem. Lett. 2019, 10, 1061–1067; (f) Xie, F.; Yang, F.; Liang, Y.; Li, L.; Xia, Y.; Jiang, F.; Liu, W.; Qi, Y.; Chowdhury, S. R.; Xie, D.; Fu, L. Investigation of stereoisomeric bisarylethenesulfonic acid esters for discovering potent and selective PTP1B inhibitors. Eur. J. Med. Chem. 2019, 164, 408–422; (g) Lei, S.; Zhang, D.; Qi, Y.; Chowdhury, S. R.; Sun, R.; Wang, J.; Du, Y.; Fu, L.; Jiang, F. Synthesis and biological evaluation of geniposide derivatives as potent and selective PTPlB inhibitors. Eur. J. Med. Chem. 2020, 205, 112508.
- 2For selected examples, see: (a) Carretero, J. C.; Ghosez, L. A new synthesis of α,β-unsaturated sulphonates and their stereoselective conversion into trans-α,β-epoxysulphonates. Tetrahedron Lett. 1987, 28, 1101–1104; (b) Chudasama, V.; Fitzmaurice, R. J.; Ahern, J. M.; Caddick, S. Dioxygen mediated hydroacylation of vinyl sulfonates and sulfones on water. Chem. Commun. 2010, 46, 133–135; (c) Mori, H.; Kudo, E.; Saito, Y.; Onuma, A.; Morishima, M. RAFT Polymerization of Vinyl Sulfonate Esters for the Controlled Synthesis of Poly(lithium vinyl sulfonate) and Sulfonated Block Copolymers. Macromolecules 2010, 43, 7021–7032; (d) Lu, J.; Ye, J.; Duan, W.-L. Palladium-Catalyzed Asymmetric Addition of Diarylphosphines to α,β-Unsaturated Sulfonic Esters for the Synthesis of Chiral Phosphine Sulfonate Compounds. Org. Lett. 2013, 15, 5016–5019.
- 3For selected examples, see: (a) Cruz, C. M.; Ortega-Munoz, M.; López-Jaramillo, F. J.; Hernández-Mateo, F.; Blanco, V.; Santoyo-González, F. Vinyl Sulfonates: A Click Function for Coupling- and-Decoupling Chemistry and their Applications. Adv. Synth. Catal. 2016, 358, 3394–3413; (b) David, A. H. G.; García-Cerezo, P.; Campaña, A. G.; Santoyo-González, F.; Blanco, V. Vinyl sulfonyl chemistry-driven unidirectional transport of a macrocycle through a [2]rotaxane. Org. Chem. Front. 2022, 9, 633–642.
- 4(a) Bordwell, F. G.; Suter, C. M.; Holbert, J. M.; Rondestvedt, C. S. Preparation of Salts of 2-Phenylethene-1-sulfonic Acid. J. Am. Chem. Soc. 1946, 68, 139–140; (b) Bordwell, F. G.; Rondestvedt, C. S. Mechanism for the Reaction of Dioxane Sulfotrioxide with Olefins. II. Sulfonation of Styrene. J. Am. Chem. Soc. 1948, 70, 2429–2433; (c) Carretero, J. C.; Demillequand, M.; Ghosez, L. Synthesis of α,β-unsaturated sulphonates via the wittig-horner reaction. Tetrahedron 1987, 43, 5125–5134.
- 5(a) Battace, A.; Zair, T.; Doucet, H.; Santelli, M. Heck Vinylations Using Vinyl Sulfide, Vinyl Sulfoxide, Vinyl Sulfone, or Vinyl Sulfonate Derivatives and Aryl Bromides Catalyzed by a Palladium Complex Derived from a Tetraphosphine. Synthesis 2006, 3495–3505; (b) Schmidt, B.; Wolf, F.; Brunner, H. Styrylsulfonates and -Sulfonamides through Pd-Catalysed Matsuda-Heck Reactions of Vinylsulfonic Acid Derivatives and Arenediazonium Salts. Eur. J. Org. Chem. 2016, 2016, 2972–2982.
- 6(a) Cala, L.; García-Pedrero, O.; Rubio-Presa, R.; Fañanás, F. J.; Rodríguez, F. Generation of alkoxysulfonyl radicals from chlorosulfates and their intramolecular capture with alkynes to obtain sultones. Chem. Commun. 2020, 56, 13425–13428; (b) Dong, X.; Jiang, W.; Hua, D.; Wang, X.; Xu, L.; Wu, X. Radical-mediated vicinal addition of alkoxysulfonyl/fluorosulfonyl and trifluoromethyl groups to aryl alkyl alkynes. Chem. Sci. 2021, 12, 11762–11768.
- 7For selected reviews, see: (a) Sperry, J. B.; Wright, D. L. The application of cathodic reductions and anodic oxidations in the synthesis of complex molecules. Chem. Soc. Rev. 2006, 35, 605–621; (b) Yoshida, J.; Kataoka, K.; Horacjada, R.; Nagaki, A. Modern Strategies in Electroorganic Synthesis. Chem. Rev. 2008, 108, 2265–2299; (c) Francke, R.; Little, R. D. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 2014, 43, 2492–2521; (d) Yan, M.; Kawamata, Y.; Baran, P. S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319; (e) Jiang, Y.; Xu, K.; Zeng, C. Use of Electrochemistry in the Synthesis of Heterocyclic Structures. Chem. Rev. 2018, 118, 4485–4540; (f) Wiebe, A.; Gieshoff, T.; Möhle, S.; Rodrigo, E.; Zirbes, M.; Waldvogel, S. R. Electrifying Organic Synthesis. Angew. Chem. Int. Ed. 2018, 57, 5594–5619; (g) Yuan, Y.; Lei, A. Electrochemical Oxidative Cross-Coupling with Hydrogen Evolution Reactions. Acc. Chem. Res. 2019, 52, 3309–3324; (h) Xiong, P.; Xu, H.-C. Chemistry with Electrochemically Generated N-Centered Radicals. Acc. Chem. Res. 2019, 52, 3339–3350; (i) Minteer, S. D.; Baran, P. S. Electrifying Synthesis: Recent Advances in the Methods, Materials, and Techniques for Organic Electrosynthesis. Acc. Chem. Res. 2020, 53, 545–546; (j) Ackermann, L. Metalla-electrocatalyzed C–H Activation by Earth-Abundant 3d Metals and Beyond. Acc. Chem. Res. 2020, 53, 84–104; (k) Jiao, K.-J.; Xing, Y.-K.; Yang, Q.-L.; Qiu, H.; Mei, T.-S. Site-Selective C-H Functionalization via Synergistic Use of Electrochemistry and Transition Metal Catalysis. Acc. Chem. Res. 2020, 53, 300–310; (l) Novaes, L. F. T.; Liu, J.; Shen, Y.; Lu, L.; Meinhardt, J. M.; Lin, S. Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev. 2021, 50, 7941–8002; (m) Murray, P. R. D.; Cox, J. H.; Chiappini, N. D.; Roos, C. B.; McLoughlin, E. A.; Hejna, B. G.; Nguyen, S. T.; Ripberger, H. H.; Ganley, J. M.; Tsui, E.; Shin, N. Y.; Koronkiewicz, B.; Qiu, G.; Knowles, R. R. Photochemical and Electrochemical Applications of Proton-Coupled Electron Transfer in Organic Synthesis. Chem. Rev. 2022, 122, 2017–2291.
- 8 Bartolomeu, A. A.; Breitschaft, F. A.; Schollmeyer, D.; Pilli, R. A.; Waldvogel, S. R. Electrochemical Multicomponent Synthesis of Alkyl Alkenesulfonates using Styrenes, SO2 and Alcohols. Chem. Eur. J. 2024, 30, e202400557.
- 9(a) Ye, S.; Qiu, G.; Wu, J. Inorganic sulfites as the sulfur dioxide surrogates in sulfonylation reactions. Chem. Commun. 2019, 55, 1013–1019; (b) Ye, S.; Yang, M.; Wu, J. Recent advances in sulfonylation reactions using potassium/sodium metabisulfite. Chem. Commun. 2020, 56, 4145–4155; (c) Zhang, L.; Cheng, X.; Zhou, Q.-L. Electrochemical Synthesis of Sulfonyl Fluorides with Triethylamine Hydrofluoride. Chin. J. Chem. 2022, 40, 1687–1692; (d) Liu, X.; Zhang, Y.; Zheng, Y.; Huang, C.; Cao, H. Controllable Construction of Vinyl Sulfones and β-Keto Selenosulfones via Selective Oxidative Sulfonylation of Alkenes. Chin. J. Chem. 2024, 42, 1367–1372.
- 10(a) Zhang, C.; Yang, M.; Qiu, Y.; Song, M.; Wang, H.; Yang, M.; Xie, W.; Wu, J.; Ye, S. Alkoxysulfonyl radical species: acquisition and transformation towards sulfonate esters through electrochemistry. Chem. Sci. 2022, 13, 11785–11791; (b) Liu, J.; Xu, J.; Mei, H.; Han, J. Electrochemical multi-component reaction of potassium metabisulfite with alkenes and alcohols enabling synthesis of sulfonate esters. Green Chem. 2022, 24, 6113–6118.
- 11(a) Liu, Y.; Zhang, X.; Lv, J.; Zhang, C.; Chang, X.; Ye, S.; Wu, J. A photocatalytic radical relay reaction of 2-methylthiolated phenylalkynones and potassium metabisulfite. Org. Chem. Front. 2022, 9, 450–455;
(b) Zhang, C.; Tang, Z.; Qiu, Y.; Tang, J.; Ye, S.; Li, Z.; Wu, J. Access to Axially Chiral Styrenes via a Photoinduced Asymmetric Radical Reaction Involving a Sulfur Dioxide Insertion. Chem. Catal. 2022, 2, 164–177;
10.1016/j.checat.2021.12.008 Google Scholar(c) He, F.-S.; Zhang, C.; Jiang, M.; Lou, L.; Wu, J.; Ye, S. Access to Chiral β-Sulfonyl Carbonyl Compounds via Photoinduced Organocatalytic Asymmetric Radical Sulfonylation with Sulfur Dioxide. Chem. Sci. 2022, 13, 8834–8839; (d) Zhang, Z.; Wang, J.; Yu, M.; Ye, S.; Wu, J. Construction of β-Amino Sulfones from Sodium Metabisulfite via a Radical 1,4-Amino Migration. Org. Lett. 2023, 25, 304–308; (e) Zhang, C.; Ye, S.; Wu, J. Asymmetric Sulfonylation from a Reaction of Cyclopropan-1-ol, Sulfur Dioxide, and 1-(Alkynyl)naphthalen-2-ol. Org. Lett. 2024, 26, 3321–3325; (f) Xiao, W.; Wang, J.; Ye, J.; Wang, H.; Wu, J.; Ye, J. Electrochemical Synthesis of Spirolactones from α-Tetralone Derivatives with Methanol as a C1 Source. Org. Lett. 2024, 26, 5016–5020.
- 12 CCDC 2368808 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.




