Enabling Access to 3-gem-Difluorovinyl Lactams via Zn-Mediated Sequential Single Electron Reductive Hydrodehalogenation
Jia Zheng
The Marine Biomedical Research Institute, School of Ocean and Tropical Medicine, Guangdong Medical University, Zhanjiang, Guangdong, 524023 China
The Marine Biomedical Research Institute of Guangdong Zhanjiang, Zhanjiang, Guangdong, 524023 China
Search for more papers by this authorXuran Liu
The Marine Biomedical Research Institute, School of Ocean and Tropical Medicine, Guangdong Medical University, Zhanjiang, Guangdong, 524023 China
Search for more papers by this authorJiawen Yin
The Marine Biomedical Research Institute, School of Ocean and Tropical Medicine, Guangdong Medical University, Zhanjiang, Guangdong, 524023 China
Search for more papers by this authorShuaikang Li
The Marine Biomedical Research Institute, School of Ocean and Tropical Medicine, Guangdong Medical University, Zhanjiang, Guangdong, 524023 China
Search for more papers by this authorJuanjuan Zhang
The Marine Biomedical Research Institute, School of Ocean and Tropical Medicine, Guangdong Medical University, Zhanjiang, Guangdong, 524023 China
Search for more papers by this authorCorresponding Author
Weigao Hu
The Marine Biomedical Research Institute, School of Ocean and Tropical Medicine, Guangdong Medical University, Zhanjiang, Guangdong, 524023 China
The Marine Biomedical Research Institute of Guangdong Zhanjiang, Zhanjiang, Guangdong, 524023 China
E-mail: [email protected]Search for more papers by this authorJia Zheng
The Marine Biomedical Research Institute, School of Ocean and Tropical Medicine, Guangdong Medical University, Zhanjiang, Guangdong, 524023 China
The Marine Biomedical Research Institute of Guangdong Zhanjiang, Zhanjiang, Guangdong, 524023 China
Search for more papers by this authorXuran Liu
The Marine Biomedical Research Institute, School of Ocean and Tropical Medicine, Guangdong Medical University, Zhanjiang, Guangdong, 524023 China
Search for more papers by this authorJiawen Yin
The Marine Biomedical Research Institute, School of Ocean and Tropical Medicine, Guangdong Medical University, Zhanjiang, Guangdong, 524023 China
Search for more papers by this authorShuaikang Li
The Marine Biomedical Research Institute, School of Ocean and Tropical Medicine, Guangdong Medical University, Zhanjiang, Guangdong, 524023 China
Search for more papers by this authorJuanjuan Zhang
The Marine Biomedical Research Institute, School of Ocean and Tropical Medicine, Guangdong Medical University, Zhanjiang, Guangdong, 524023 China
Search for more papers by this authorCorresponding Author
Weigao Hu
The Marine Biomedical Research Institute, School of Ocean and Tropical Medicine, Guangdong Medical University, Zhanjiang, Guangdong, 524023 China
The Marine Biomedical Research Institute of Guangdong Zhanjiang, Zhanjiang, Guangdong, 524023 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
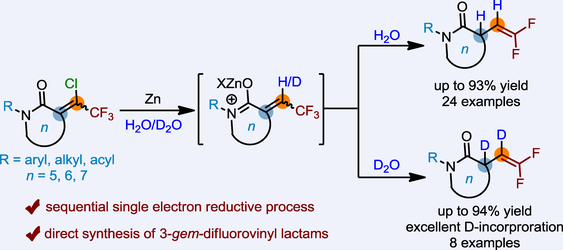
Herein, we describe a direct route for the synthesis of 3-gem-difluorovinyl lactams through Zn-mediated reductive hydrodehalogenation. Importantly, by using inexpensive deuterium oxide (D2O), the high value-added vicinal dideuterated gem-difluoroalkenes with excellent deuterium (D) incorporation were prepared. Mechanism studies indicated a successive single electron transfer process: the reaction initially undergoes hydrodechlorination to give the intermediate α-trifluoromethylidene lactams, which are then activated by the in-situ generated zinc cations and reduced to the desired product via hydrodefluorination.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400718-sup-0001-supinfo.pdfPDF document, 9 MB |
Appendix S1: Supplement information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see: (a) Lu, M.-Z.; Goh, J.; Maraswami, M.; Jia, Z.; Tian, J.-S.; Loh, T. -P. Recent Advances in Alkenyl sp2 C–H and C–F Bond Functionalizations: Scope, Mechanism, and Applications. Chem. Rev. 2022, 122, 17479–17646; (b) Liu, C.; Zeng, H.; Zhu, C. L.; Jiang, H. F. Recent advances in three-component difunctionalization of gem-difluoroalkenes. Chem. Commun. 2020, 56, 10442–10452; (c) Koley, S.; Altman, R. A. Recent Advances in Transition Metal-Catalyzed Functionalization of gem-Difluoroalkenes. Isr. J. Chem. 2020, 60, 313–339.
- 2(a) Bobek, M.; Kavai, I.; Clercq, E. D. Synthesis and Biological Activity of 5-(2,2-difluorovinyl)-2’-deoxyuridine. J. Med. Chem. 1987, 30, 1494–1497; (b) Maring, C. J.; Stoll, V. S.; Zhao, C.; Sun, M. H.; Krueger, A. C.; Stewart, K. D.; Madigan, D. L.; Kati, W. M.; Xu, Y. B.; Carrick, R. J.; Montgomery, D. A.; Kempf-Grote, A.; Marsh, K. C.; Molla, A.; Steffy, K. R.; Sham, H. L.; Graeme Laver, W.; Gu, Y. G.; Kempf, D. J.; Kohlbrenner, W. E. Structure-Based Characterization and Optimization of Novel Hydrophobic Binding Interactions in a Series of Pyrrolidine Influenza Neuraminidase Inhibitors. J. Med. Chem. 2005, 48, 3980–3990; (c) Martella, G.; Bonsi, P.; Sciamanna, G.; Platania, P.; Madeo, G.; Tassone, A.; Cuomo, D.; Pisani, A. Seletracetam (ucb 44212) inhibits high-voltage–activated Ca2+ currents and intracellular Ca2+ increase in rat cortical neurons in vitro. Epilepsia 2009, 50, 702–710.
- 3(a) Watterson, A. C.; Shama, S. A. Photochemistry of acid hydrazides. Determination of modes of reaction and identification of photoproducts. J. Org. Chem. 1975, 40, 19–24; (b) Zheng, J.; Cai, J.; Lin, J. H; Guo, Y.; Xiao, J. C. Synthesis and decarboxylative Wittig reaction of difluoromethylene phosphobetaine. Chem. Commun. 2013, 49, 7513–7515.
- 4(a) Obayashi, M.; Ito, E.; Matsui, K.; Kondo, K. (Diethylphosphinyl)difluoromethyllithium.—Preparation and synthetic application—. Tetrahedron Lett. 1982, 23, 2323–2326; (b) McCarthy, J. R.; Matthews, D. P.; Stemerick, D. M.; Huber, E. W.; Bey, P.; Lippert, B. J.; Snyder, R. D.; Sunkara, P. S. Stereospecific method to (E) and (Z) terminal fluoroolefins and its application to the synthesis of 2’-deoxy-2’-fluoromethylenenucleosides as potential inhibitors of ribonucleoside diphosphate reductase. J. Am. Chem. Soc. 1991, 113, 7439–7440.
- 5(a) Prakash, G. K. S.; Wang, Y.; Hu, J. B.; Olah, G. A. Nucleophilic difluoromethylation and difluoromethylenation using bromodifluoromethyl phenyl sulfone. J. Fluorine Chem. 2005, 126, 1361–1367; (b) Zhao, Y. C.; Huang, W. Z.; Zhu, L. G.; Hu, J. B. Difluoromethyl 2-Pyridyl Sulfone: A New gem-Difluoroolefination Reagent for Aldehydes and Ketones. Org. Lett. 2010, 12, 1444–1447; (c) Gao, B.; Zhao, Y. C.; Hu, M. Y.; Ni, C. F.; Hu, J. B. gem-Difluoroolefination of diaryl ketones and enolizable aldehydes with difluoromethyl 2-pyridyl sulfone: new insights into the Julia–Kocienski reaction. Chem. Eur. J. 2014, 20, 7803–7810.
- 6(a) Zhao, F.; Zhou, W. L.; Zuo, Z. Recent advances in the synthesis of difluorinated architectures from trifluoromethyl groups. Adv. Synth. Catal. 2022, 364, 234–267; (b) Chelucci, G. Synthesis and metal- catalyzed reactions of gem-dihalovinyl systems. Chem. Rev. 2012, 112, 1344–1462.
- 7(a) Bégué, J.-P.; Bonnet-Delpon, D.; Rock, M. H. A concise synthesis of functionalised gem-difluoroalkenes, via the addition of organolithium reagents to α-trifluoromethylstyrene. Tetrahedron Lett. 1995, 36, 5003–5006; (b) Cai, Y. Y.; Hao, Z.; Zhu, C. L.; Liu, C.; Liu, G. Y.; Jiang, H. F. Double allylic defluorinative alkylation of 1,1-bisnucleophiles with (trifluoromethyl)alkenes: construction of all-carbon quaternary centers. Org. Chem. Front. 2020, 7, 1260–1265.
- 8(a) Gao, X. T.; Zhang, Z.; Wang, X.; Tian, J. S.; Xie, S. L.; Zhou, F.; Zhou, J. Direct electrochemical defluorinative carboxylation of α-CF3 alkenes with carbon dioxide. Chem. Sci. 2020, 11, 10414–10420; (b) He, Y. W.; Anand, D.; Sun, Z. C.; Zhou, L. Visible-Light-Promoted Redox Neutral γ,γ-Difluoroallylation of Cycloketone Oxime Ethers with Trifluoromethyl Alkenes via C–C and C–F Bond Cleavage. Org. Lett. 2019, 21, 3769–3773; (c) Tian, J. B.; Zhou, L. Photoredox radical/ polar crossover enables C–H gem-difunctionalization of 1,3-benzodioxoles for the synthesis of monofluorocyclohexenes. Chem. Sci. 2023, 14, 6045–6051.
- 9(a) Yue, W. J.; Day, C. S.; Martin, R. Site-Selective Defluorinative sp3 C–H Alkylation of Secondary Amides. J. Am. Chem. Soc. 2021, 143, 6395–6400; (b) Huang, Y. H.; Hayashi, T. Rhodium-Catalyzed Asymmetric Arylation/Defluorination of 1-(Trifluoromethyl) alkenes Forming Enantioenriched 1,1-Difluoroalkenes. J. Am. Chem. Soc. 2016, 138, 12340–12343; (c) Wang, M. Y.; Pu, X. H.; Zhao, Y. F.; Wang, P. P.; Li, Z. X.; Zhu, C. D.; Shi, Z. Z. Enantioselective Copper-Catalyzed Defluoroalkylation Using Arylboronate-Activated Alkyl Grignard Reagents. J. Am. Chem. Soc. 2018, 140, 9061–9065; (d) Ping, Y. Y.; Li, X.; Pan, Q.; Kong, W. Q. Ni-Catalyzed Divergent Synthesis of 2-Benzazepine Derivatives via Tunable Cyclization and 1,4-Acyl Transfer Triggered by Amide N-C Bond Cleavage. Angew. Chem. Int. Ed. 2022, 61, e202201574; (e) Ping, Y. Y.; Pan, Q.; Guo, Y.; Liu, Y. L.; Li, X.; Wang, M. Y.; Kong, W. Q. Switchable 1,2-Rearrangement Enables Expedient Synthesis of Structurally Diverse Fluorine-Containing Scaffolds. J. Am. Chem. Soc. 2022, 144, 11626–11637.
- 10 Xu, P.; Daniliuc, C. G.; Bergander, K.; Stein, C.; Studer, A. Synthesis of Five-Membered Ring Systems Bearing gem-Difluoroalkenyl and Monofluoroalkenyl Substituents via Radical β-Bromo Fragmentation. ACS Catal. 2022, 12, 11934–11941.
- 11 Wang, K.; Chen, J. C.; Liu, W. F.; Kong, W. Q. Nickel-Catalyzed Defluorinative Asymmetric Cyclization of Fluoroalkyl-Substituted 1,6-Enynes for the Synthesis of Seletracetam. Angew. Chem. Int. Ed. 2022, 61, e202212664.
- 12(a) Leriche, C.; He, X. M.; Chang, C. W. T.; Liu, H. W. Reversal of the apparent regiospecificity of NAD(P)H-dependent hydride transfer: the properties of the difluoromethylene group, a carbonyl mimic. J. Am. Chem. Soc. 2003, 125, 6348–6349; (b) Magueur, G.; Crousse, B.; Ourévitch, M.; Bonnet-Delpon, D.; Bégué, J.-P. Fluoro-artemisinins: when a gem-difluoroethylene replaces a carbonyl group. J. Fluor. Chem. 2006, 127, 637–642.
- 13(a) Cheng, R.; Sang, Y. Q.; Gao, X.; Zhang, S.; Xue, X. S.; Zhang, X. G. Highly γ-Selective Arylation and Carbonylative Arylation of 3-Bromo- 3,3-difluoropropene via Nickel Catalysis. Angew. Chem. Int. Ed. 2021, 60, 12386–12391; (b) Wu, X. T.; Xie, F.; Gridnev, I. D.; Zhang, W. B. A Copper-Catalyzed Reductive Defluorination of β-Trifluoromethylated Enones via Oxidative Homocoupling of Grignard Reagents. Org. Lett. 2018, 20, 1638–1642.
- 14 An, S. Y.; Zhang, J. L.; Jiang, G. X. Synthesis of gem-Difluoroalkenes via a Sequence of Hydroboration and 1,2-Elimination of α,β-Unsaturated Carbonyls. Synlett 2021, 32, 91–94.
- 15 Amii, H.; Uneyama, K. C-F Bond Activation in Organic Synthesis. Chem. Rev. 2009, 109, 2119–2183.




