Enhancing Oxygen Reduction Reaction Electrocatalytic Performance of Nickel-Nitrogen-Carbon Catalysts through Coordination Environment Engineering†
Hui-Jian Zou
School of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorYan Leng
Research Center for Analysis and Measurement, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorChen-Shuang Yin
School of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorXikun Yang
Research Center for Analysis and Measurement, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorCorresponding Author
Chun-Gang Min
School of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Research Center for Analysis and Measurement, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
E-mail: [email protected] (C. G. Min); [email protected] (F. Tan); [email protected] (A. M. Ren)Search for more papers by this authorCorresponding Author
Feng Tan
School of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Kunming Sino-Platinum Metals Catalyst Co., Ltd., Kunming, Yunnan, 650106 China
Yunnan Precious Metals Lab. Co., Ltd., Kunming, Yunnan, 650106 China
E-mail: [email protected] (C. G. Min); [email protected] (F. Tan); [email protected] (A. M. Ren)Search for more papers by this authorCorresponding Author
Ai-Min Ren
Institute of Theoretical Chemistry, College of Chemistry, Jilin University, Changchun, Jilin, 130023 China
E-mail: [email protected] (C. G. Min); [email protected] (F. Tan); [email protected] (A. M. Ren)Search for more papers by this authorHui-Jian Zou
School of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorYan Leng
Research Center for Analysis and Measurement, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorChen-Shuang Yin
School of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorXikun Yang
Research Center for Analysis and Measurement, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorCorresponding Author
Chun-Gang Min
School of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Research Center for Analysis and Measurement, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
E-mail: [email protected] (C. G. Min); [email protected] (F. Tan); [email protected] (A. M. Ren)Search for more papers by this authorCorresponding Author
Feng Tan
School of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Kunming Sino-Platinum Metals Catalyst Co., Ltd., Kunming, Yunnan, 650106 China
Yunnan Precious Metals Lab. Co., Ltd., Kunming, Yunnan, 650106 China
E-mail: [email protected] (C. G. Min); [email protected] (F. Tan); [email protected] (A. M. Ren)Search for more papers by this authorCorresponding Author
Ai-Min Ren
Institute of Theoretical Chemistry, College of Chemistry, Jilin University, Changchun, Jilin, 130023 China
E-mail: [email protected] (C. G. Min); [email protected] (F. Tan); [email protected] (A. M. Ren)Search for more papers by this author† Dedicated to the Special Issue of Nickel Catalysis.
Comprehensive Summary
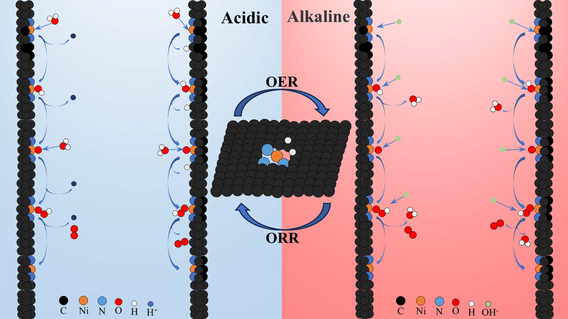
Single-atom catalysts (SACs) have attracted significant attention due to their high atomic utilization and tunable coordination environment. However, the catalytic mechanisms related to the active center and coordination environment remain unclear. In this study, we systematically investigated the oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) catalytic activities of NiN4, NiN3, NiN3H2, NiN4X, NiN3X, and NiN3H2X (X denotes axial ligand) through density functional theory (DFT) calculations. This study unveils two distinct reaction pathways for ORR and OER, involving proton-electron pairs adsorbed from both the solution and the catalyst surface. The overpotential is the key parameter to evaluate the catalytic performance when proton-electron pairs are adsorbed from the solution. NiN3 and NiN3H2 show promise as pH-universal bifunctional electrocatalysts for both ORR and OER. On the other hand, when proton-electron pairs are adsorbed from the catalyst surface, the reaction energy barrier becomes the crucial metric for assessing catalytic activity. Our investigation reveals that NiN3H2 consistently exhibits optimal ORR activity across a wide pH range, regardless of the source of proton-electron pair (solvent or catalyst surface).
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400769-sup-0001-supinfo.pdfPDF document, 5.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Gao, Y.; Mei, B.; Wu, Y.; Zhao, Q.; Bao, Z.; Qin, H.; Xu, Y.; Lv, H.; Peng, X.; He, Y.; Luo, T.; Yao, R.; Zhang, W.; Lei, H.; Cao, R. A Cobalt(III) Corrole with a Tethered Imidazole for Boosted Electrocatalytic Oxygen Reduction Reaction. Chin. J. Chem. 2023, 41, 2866–2872.
- 2 Li, J.; Xia, W.; Tang, J.; Gao, Y.; Jiang, C.; Jia, Y.; Chen, T.; Hou, Z.; Qi, R.; Jiang, D.; Asahi, T.; Xu, X.; Wang, T.; He, J.; Yamauchi, Y. Metal-Organic Framework-Derived Graphene Mesh: A Robust Scaffold for Highly Exposed Fe-N4 Active Sites toward an Excellent Oxygen Reduction Catalyst in Acid Media. J. Am. Chem. Soc. 2022, 144, 9280–9291.
- 3 Pang, R.; Xia, H.; Li, J.; Guo, S.; Wang, E. Recent Developments of Atomically Dispersed Metal Electrocatalysts for Oxygen Reduction Reaction. Chin. J. Chem. 2023, 41, 581–598.
- 4 Li, Y.; Ren, P.; Lu, X.; Zhang, J.; Yang, P.; Yang, X.; Wang, G.; Liu, A.; Wu, G.; An, M. Elucidating the Role of P on Mn- and N-doped Graphene Catalysts in Promoting Oxygen Reduction: Density Functional Theory Studies. SusMat 2023, 3, 390–401.
- 5 Zhao, Y.; Liu, Y. C.; Miao, B. Q.; Ding, Y.; Jin, P. J.; Chen, Y. One-Dimensional Rhodium-Nickel Alloy Assemblies with Nanodendrite Subunits for Alkaline Methanol Oxidation. Chin. J. Struct. Chem. 2022, 41, 2204040–2204045.
- 6 Sun, B.; Jiang, Y. C.; Hong, Q. L.; Liu, X.; Li, F. M.; Li, D. S.; Yang, Y.; Chen, Y. Pt-Te Alloy Nanowires towards Formic Acid Electrooxidation Reaction. J. Energy Chem. 2023, 85, 481–489.
- 7 Miao, B. Q.; Yuan, Z. H.; Liu, X. L.; Ai, X.; Zhao, G. T.; Chen, P.; Jin, P. J.; Chen, Y. Tensile Strain and Interatomic Orbital Hybridization Effects Boost the Electrocatalytic Performance of Intermetallic Pd3Pb Nanowires for Ethanol Electrooxidation. Chin. J. Chem. 2024, 42, 2633–2640.
- 8 Zhan, F.; Hu, K.-S.; Mai, J.-H.; Zhang, L.-S.; Zhang, Z.-G.; He, H.; Liu, X.-H. Recent Progress of Pt-Based Oxygen Reduction Reaction Catalysts for Proton Exchange Membrane Fuel Cells. Rare Met. 2024, 43, 2444–2468.
- 9 Chen, Q.; Zhang, Z.; Zhang, R. Q.; Hu, M. C.; Shi, L.; Yao, Z. H. Recent Progress of Non-Pt Catalysts for Oxygen Reduction Reaction in Fuel Cells. Processes 2023, 11, 361.
- 10 Bai, J.; Zhou, W. K.; Xu, J. N.; Zhou, P.; Deng, Y. Y.; Xiang, M.; Xiang, D. S.; Su, Y. Q. RuO2 Catalysts for Electrocatalytic Oxygen Evolution in Acidic Media: Mechanism, Activity Promotion Strategy and Research Progress. Molecules 2024, 29, 537.
- 11 Wang, J.; Zheng, M.; Zhao, X.; Fan, W. Structure-Performance Descriptors and the Role of the Axial Oxygen Atom on M-N4-C Single-Atom Catalysts for Electrochemical CO2 Reduction. ACS Catal. 2022, 12, 5441–5454.
- 12
Hu, S.; Zhu, M. Recent Advances in Carbon-Based Non-Noble Single-Atom Catalysts for Rechargeable Zinc–Air Batteries. Curr. Opin. Chem. Eng. 2023, 41, 100926.
10.1016/j.coche.2023.100926 Google Scholar
- 13 Zou, L.; Wei, Y.; Hou, C.; Li, C.; Xu, Q. Single-Atom Catalysts Derived from Metal-Organic Frameworks for Electrochemical Applications. Small 2021, 17, 2004809.
- 14 Saetta, C.; Di Liberto, G.; Pacchioni, G. Water Splitting on a Pt1/C3N4 Single Atom Catalyst: A Modeling Approach. Top. Catal. 2023, 66, 1120–1128.
- 15 Zhou, Y.; Gao, G.; Kang, J.; Chu, W.; Wang, L. Transition Metal- Embedded Two-Dimensional C3N as a Highly Active Electrocatalyst for Oxygen Evolution and Reduction Reactions. J. Mater. Chem. A Mater. 2019, 7, 12050–12059.
- 16 Xiao, M.; Xing, Z.; Jin, Z.; Liu, C.; Ge, J.; Zhu, J.; Wang, Y.; Zhao, X.; Chen, Z. Preferentially Engineering FeN4 Edge Sites onto Graphitic Nanosheets for Highly Active and Durable Oxygen Electrocatalysis in Rechargeable Zn-Air Batteries. Adv. Mater. 2020, 32, e2004900.
- 17 Shen, H.; Thomas, T.; Rasaki, S.; Saad, A.; Hu, C.; Wang, J.; Yang, M. Oxygen Reduction Reactions of Fe-N-C Catalysts: Current Status and the Way Forward. Electrochem. Energy Rev. 2019, 2, 252–276.
- 18 Kumar, P.; Kannimuthu, K.; Zeraati, A. S.; Roy, S.; Wang, X.; Wang, X.; Samanta, S.; Miller, K. A.; Molina, M.; Trivedi, D.; Abed, J.; Campos Mata, M. A.; Al-Mahayni, H.; Baltrusaitis, J.; Shimizu, G.; Wu, Y. A.; Seifitokaldani, A.; Sargent, E. H.; Ajayan, P. M.; Hu, J.; Kibria, M. G. High-Density Cobalt Single-Atom Catalysts for Enhanced Oxygen Evolution Reaction. J. Am. Chem. Soc. 2023, 145, 8052–8063.
- 19 Fei, H.; Dong, J.; Feng, Y.; Allen, C.; Wan, C.; Volosskiy, B.; Li, M.; Zhao, Z.; Wang, Y.; Sun, H.; An, P.; Chen, W.; Guo, Z.; Lee, C.; Chen, D.; Shakir, I.; Liu, M.; Hu, T.; Li, Y.; Kirkland, A.; Duan, X.; Huang, Y. General Synthesis and Definitive Structural Identification of MN4C4 Single-Atom Catalysts with Tunable Electrocatalytic Activities. Nat. Catal. 2018, 1, 63–72.
- 20 Li, T.; Deng, H.; Liu, J.; Jin, C.; Song, Y.; Wang, F. First-Row Transition Metals and Nitrogen Co-Doped Carbon Nanotubes: The Exact Origin of the Enhanced Activity for Oxygen Reduction Reaction. Carbon 2019, 143, 859–868.
- 21 Shi, Z.; Yang, W.; Gu, Y.; Liao, T.; Sun, Z. Metal-Nitrogen-Doped Carbon Materials as Highly Efficient Catalysts: Progress and Rational Design. Adv. Sci. 2020, 7, 2001069.
- 22 Liu, J.; Xiao, J.; Luo, B.; Tian, E.; Waterhouse, G. Central Metal and Ligand Effects on Oxygen Electrocatalysis over 3d Transition Metal Single-Atom Catalysts: A Theoretical Investigation. Chem. Eng. J. 2022, 427, 132038.
- 23 Xue, Z.; Zhang, X.; Qin, J.; Liu, R. TMN4 Complex Embedded Graphene as Bifunctional Electrocatalysts for High Efficiency OER/ORR. J. Energy Chem. 2021, 55, 437–443.
- 24 Yan, T.; Li, X.; Li, Z.; Zhao, J. Rationally Designed Metal-N-C/MoS2 Heterostructures as Bifunctional Oxygen Electrocatalysts: A Computational Study. Appl. Surf. Sci. 2022, 606, 154969.
- 25 Xiao, B. B.; Yang, L.; Liu, H. Y.; Jiang, X. B.; Aleksandr, B.; Song, E. H.; Jiang, Q. Designing Fluorographene with FeN4 and CoN4 Moieties for Oxygen Electrode Reaction: A Density Functional Theory Study. Appl. Surf. Sci. 2021, 537, 147846.
- 26 Cipriano, L. A.; Di Liberto, G.; Pacchioni, G. Superoxo and Peroxo Complexes on Single-Atom Catalysts: Impact on the Oxygen Evolution Reaction. ACS Catal. 2022, 12, 11682–11691.
- 27 Sun, H.; Ma, Y.; Zhang, Q.; Su, C. Engineering the Local Coordination Environment of Single-Atom Catalysts and Their Applications in Photocatalytic Water Splitting: A Review. Trans. Tianjin Univ. 2021, 27, 313–330.
- 28 Liu, X.; Liu, Y.; Yang, W.; Feng, X.; Wang, B. Controlled Modification of Axial Coordination for Transition-Metal Single-Atom Electrocatalyst. Chem.-Eur. J. 2022, 28, e202201471.
- 29 Zhang, W.; Pan, J. K.; Yu, Y. F.; Zhang, X. J.; Wang, J. H.; Chen, W. X.; Zhuang, G. L. Correlation of the Spin State and Catalytic Property of M-N4 Single-Atom Catalysts in Oxygen Reduction Reactions. Phys. Chem. Chem. Phys. 2023, 25, 11673–11683.
- 30 Yin, Y.; Liao, S.; Lin, H.; Liu, F.; Liu, Y.; Xiao, Y.; Min, Y. A Facile and Efficient Ni-N-C Electrocatalyst Derived from Superabsorbent Resin for Oxygen Evolution Reaction. J. Electrochem. Soc. 2021, 168, 046519
- 31 Li, Y.; Wu, Z.; Lu, P.; Wang, X.; Liu, W.; Liu, Z.; Ma, J.; Ren, W.; Jiang, Z.; Bao, X. High-Valence Nickel Single-Atom Catalysts Coordinated to Oxygen Sites for Extraordinarily Activating Oxygen Evolution Reaction. Adv. Sci. 2020, 7, 1903089.
- 32 Xin, C.; Shang, W.; Hu, J.; Zhu, C.; Guo, J.; Zhang, J.; Dong, H.; Liu, W.; Shi, Y. Integration of Morphology and Electronic Structure Modulation on Atomic Iron-Nitrogen-Carbon Catalysts for Highly Efficient Oxygen Reduction. Adv. Funct. Mater. 2021, 32, 2108345.
- 33 Chen, X.; Zhang, Y. Z.; Zhao, X. Y.; Yu, H.; Zhang, H. Effect of the Axial Halogen Ligand on the Oxygen Reduction Reaction Performance of Transition Metal-Nitrogen-Carbon Catalysts. J. Phys. Chem. C 2023, 127, 14107–14116.
- 34 Hu, L. Y.; Dai, C. L.; Chen, L. W.; Zhu, Y. H.; Hao, Y. C.; Zhang, Q. H.; Gu, L.; Feng, X.; Yuan, S.; Wang, L.; Wang, B. Metal-Triazolate-Framework-Derived FeN4Cl1 Single-Atom Catalysts with Hierarchical Porosity for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2021, 60, 27324–27329.
- 35 Fei, H.; Dong, J.; Chen, D.; Hu, T.; Duan, X.; Shakir, I.; Huang, Y.; Duan, X. Single Atom Electrocatalysts Supported on Graphene or Graphene- Like Carbons. Chem. Soc. Rev. 2019, 48, 5207–5241.
- 36 Li, W.; Guo, B.; Zhang, K.; Zhang, H.; Bu, K.; Chen, H.; Feng, X. A Co-Axial Structure Composed of RuO2 on Defective N-doped Carbon Nanotubes as a Highly Efficient Electrocatalyst for Overall Water Splitting. Inorg. Chem. Front. 2024, 11, 745–755.
- 37 Wan, W.; Triana, C.; Lan, J.; Li, J.; Allen, C.; Zhao, Y.; Iannuzzi, M.; Patzke, G. Bifunctional Single Atom Electrocatalysts: Coordination- Performance Correlations and Reaction Pathways. ACS Nano 2020, 14, 13279–13293.
- 38 Zhang, Q.; Guan, J. Single-Atom Catalysts for Electrocatalytic Applications. Adv. Funct. Mater. 2020, 30, 2000768.
- 39 Zhang, F.; Liu, H.; Tao, F.; Wang, X.; Cao, X.; Hu, W. Tunable Electric and Magnetic Properties of Transition Metal@NxCy-Graphene Materials by Different Metal and Defect Types. Chem. Asian J. 2021, 16, 3230–3235.
- 40 Guo, M.; Ji, M.; Cui, W. Theoretical Investigation of HER/OER/ORR Catalytic Activity of Single Atom-Decorated Graphyne by DFT and Comparative DOS Analyses. Appl. Surf. Sci. 2022, 592, 153237.
- 41 Ha, M.; Kim, D.; Umer, M.; Gladkikh, V.; Myung, C.; Kim, K. Tuning Metal Single Atoms Embedded in NxCy Moieties toward High-Performance Electrocatalysis. Energy Environ. Sci. 2021, 14, 3455–3468.
- 42 Dong, F.; Wu, M.; Chen, Z.; Liu, X.; Zhang, G.; Qiao, J.; Sun, S. Atomically Dispersed Transition Metal-Nitrogen-Carbon Bifunctional Oxygen Electrocatalysts for Zinc-Air Batteries: Recent Advances and Future Perspectives. Nanomicro Lett. 2021, 14, 36.
- 43 Kattel, S.; Atanassov, P.; Kiefer, B. Stability, Electronic and Magnetic Properties of In-Plane Defects in Graphene: A First-Principles Study. J. Phys. Chem. C 2012, 116, 8161–8166.
- 44 Yin, C. S.; Leng, Y.; Yang, X. K.; Min, C. G.; Ren, A. M. Unraveling the Mechanism of Pyrrole and N-Defect Regulating CoN4 Single Atom Catalysts as a pH-Universal Bifunctional Electrocatalyst for OER and ORR. Appl. Surf. Sci. 2024, 643, 158605.
- 45 Yin, C. S.; Leng, Y.; Yang, X. K.; Min, C. G.; Ren, A. M.; Liu, G. The Effect of N-Defect and Axial Halogen Atom on Electrocatalytic Oxygen Reduction Reaction Ativity of FeN4 Sngle-Atom Catalysts: A Density Functional Theory Study. ChemistrySelect 2024, 9, e202304408.
- 46 Sun, P.; Qiao, Z.; Wang, S.; Li, D.; Liu, X.; Zhang, Q.; Zheng, L.; Zhuang, Z.; Cao, D. Atomically Dispersed Zn-Pyrrolic-N4 Cathode Catalysts for Hydrogen Fuel Cells. Angew. Chem. Int. Ed. 2023, 62, e202216041.
- 47 Chen, R.; Liu, W.; Sang, Z.; Jia, J.; Li, Z.; Nie, J.; Jiang, Q.; Mao, Z.; Guo, B.; Wang, Q.; Hou, F.; Yin, L.; Yang, D. a.; Liang, J. Identification of the Highly Active Zn-N4 sites with Pyrrole/Pyridine-N Synergistic Coordination by dz2+s-Band Center for Electrocatalytic H2O2 Production. J. Energy Chem. 2024, 98, 105–113.
- 48 Ha, M.; Kim, D.; Umer, M.; Gladkikh, V.; Myung, C.; Kim, K. Tuning Metal Single Atoms Embedded in NxCy Moieties toward High-Performance Electrocatalysis. Energy Environ. Sci. 2021, 14, 3455–3468.
- 49 Wang, Y.; Wang, R.; Li, Y. Atomically Dispersed Transition Metal-N4 Doped Graphene as a Li O Nucleation Site in Nonaqueous Lithium- Oxygen Batteries. Electrochim. Acta 2022, 422, 140554.
- 50 Zhang, C.; Dai, Y.; Sun, Q.; Ye, C.; Lu, R.; Zhou, Y.; Zhao, Y. Strategy to Weaken the Oxygen Adsorption on Single-Atom Catalysts towards Oxygen-Involved Reactions. Mater. Today Adv. 2022, 16, 100280.
- 51 Wang, Q.; Ji, Y.; Lei, Y.; Wang, Y.; Wang, Y.; Li, Y.; Wang, S. Pyridinic-N-Dominated Doped Defective Graphene as a Superior Oxygen Electrocatalyst for Ultrahigh-Energy-Density Zn–air Batteries. ACS Energy Lett. 2018, 3, 1183–1191.
- 52 Cao, X. R.; Li, X. F.; Hu, W. Tunable Electronic and Magnetic Properties of Graphene-Embedded Transition Metal-N4 Complexes: Insight From First-Principles Calculations. Chem.-Asian J. 2018, 13, 3239–3245.
- 53 Wang, Y.; Huang, X.; Fu, H.; Shang, J. Theoretically Revealing the Activity Origin of the Hydrogen Evolution Reaction on Carbon-Based Single-Atom Catalysts and Finding Ideal Catalysts for Water Splitting. J. Mater. Chem. A Mater. 2022, 10, 24362–24372.
- 54 Barlocco, I.; Cipriano, L. A.; Di Liberto, G.; Pacchioni, G. Does the Oxygen Evolution Reaction Follow the Classical OH*, O*, OOH* Path on Single Atom Catalysts? J. Catal. 2023, 417, 351–359.
- 55 Xiao, G.; Lu, R.; Liu, J.; Liao, X.; Wang, Z.; Zhao, Y. Coordination Environments Tune the Activity of Oxygen Catalysis on Single Atom Catalysts: A Computational Study. Nano Res. 2021, 15, 3073–3081.
- 56 Mao, X.; Ling, C.; Tang, C.; Yan, C.; Zhu, Z.; Du, A. Predicting a New Class of Metal-Organic Frameworks as Efficient Catalyst for Bi-functional Oxygen Evolution/Reduction Reactions. J. Catal. 2018, 367, 206–211.
- 57 Fu, Z.; Ling, C.; Wang, J. A Ti3C2O2 Supported Single Atom, Trifunctional Catalyst for Electrochemical Reactions. J. Mater. Chem. A Mater. 2020, 8, 7801–7807.
- 58 Ling, C.; Shi, L.; Ouyang, Y.; Zeng, X.; Wang, J. Nanosheet Supported Single-Metal Atom Bifunctional Catalyst for Overall Water Splitting. Nano Lett. 2017, 17, 5133–5139.
- 59 Liang, Z.; Luo, M.; Chen, M.; Qi, X.; Liu, J.; Liu, C.; Peera, S.; Liang, T. Exploring the Oxygen Electrode Bifunctional Activity of Ni-N-C-Doped Graphene Systems with N, C Coordination and OH Ligand Effects. J. Mater. Chem. A Mater. 2020, 8, 20453–20462.
- 60 Rossmeisl, J.; Qu, Z. W.; Zhu, H.; Kroes, G. J.; Nørskov, J. K. Electrolysis of Water on Oxide Surfaces. J. Electroanal. Chem. 2007, 607, 83–89.
- 61 Duan, Z.; Wang, G. Comparison of Reaction Energetics for Oxygen Reduction Reactions on Pt(100), Pt(111), Pt/Ni(100), and Pt/Ni(111) Surfaces: A First-Principles Study. J. Phys. Chem. C 2013, 117, 6284–6292.
- 62 Nørskov, J. K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J. R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892.
- 63 Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J. K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312.
- 64 Wang, Y.; Wang, M.; Lu, Z.; Ma, D.; Jia, Y. Enabling Multifunctional Electrocatalysts by Modifying the Basal Plane of Unifunctional 1T’-MoS2 with Anchored Transition Metal Single Atoms. Nanoscale 2021, 13, 13390–13400.
- 65 Zhou, Y.; Gao, G.; Chu, W.; Wang, L. Computational Screening of Transition Metal-Doped Phthalocyanine Monolayers for Oxygen Evolution and Reduction. Nanoscale Adv. 2020, 2, 710–716.
- 66 Lu, R.; Quan, C.; Zhang, C.; He, Q.; Liao, X.; Wang, Z.; Zhao, Y. Establishing a Theoretical Insight for Penta-Coordinated Iron-Nitrogen-Carbon Catalysts toward Oxygen Reaction. Nano Res. 2022, 15, 6067–6075.
- 67 Kohn, W.; Sham, L. J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138.
- 68 Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comp. Mater. Sci. 1996, 6, 15–50.
- 69 Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B Condens. Matter 1996, 54, 11169–11186.
- 70 Blochl, P. E. Projector Augmented-Wave Method. Phys. Rev. B Condens. Matter 1994, 50, 17953–17979.
- 71 Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775.
- 72 Kresse, G.; Hafner, J. Ab Initio Molecular-Dynamics Simulation of the Liquid-Metal-Amorphous-Semiconductor Transition in Germanium. Phys. Rev. B Condens. Matter 1994, 49, 14251–14269.
- 73 Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
- 74 Monkhorst, H. J.; Pack, J. D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192.
- 75 Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104.
- 76 Grimme, S. Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799.
- 77 Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465.
- 78 Tang, W.; Sanville, E.; Henkelman, G. A Grid-Based Bader Analysis Algorithm without Lattice Bias. J. Phys. Condens. Matter 2009, 21, 084204.
- 79 Henkelman, G.; Uberuaga, B.; Jónsson, H. A Climbing Image Nudged Elastic Band Method for Finding Saddle Points and Minimum Energy Paths. J. Chem. Phys. 2000, 113, 9901–9904.
- 80 Krukau, A. V.; Vydrov, O. A.; Izmaylov, A. F.; Scuseria, G. E. Influence of the Exchange Screening Parameter on the Performance of Screened Hybrid Functionals. J. Chem. Phys. 2006, 125, 224106.
- 81 Hu, R.; Li, Y.; Wang, F.; Shang, J. Rational Prediction of Multifunctional Bilayer Single Atom Catalysts for the Hydrogen Evolution, Oxygen Evolution and Oxygen Reduction Reactions. Nanoscale 2020, 12, 20413–20424.
- 82 Nose, S. A. Constant Temperature Molecular Dynamics Methods. Prog. Theor. Phys. Suppl. 1991, 103, 1–46.
- 83 Nosé, S. A Unified Formulation of the Constant Temperature Molecular Dynamics Methods. J. Chem. Phys. 1984, 81, 511–519.
- 84 Hoover, W. G. Canonical Dynamics: Equilibrium Phase-Space Distributions. Phys. Rev. A Gen. Phys. 1985, 31, 1695–1697.
- 85 Bylander, D. M.; Kleinman, L. Energy Fluctuations Induced by the Nose Thermostat. Phys. Rev. B Condens. Matter 1992, 46, 13756–13761.
- 86 Momma, K.; Izumi, F. VESTA: A Three-Dimensional Visualization System for Electronic and Structural Analysis. J. Appl. Crystallogr. 2008, 41, 653–658.




