Modular Access to Chiral 1,3-Substituted Fragments via Nickel-Catalyzed Arylboration Reaction†
Yang Bao
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorTong Yao
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorWeiyu Kong
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorYangyang Li
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Ying Fu
College of Arts and Sciences, Northeast Agricultural University, Harbin, Heilongjiang, 150030 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Dong Wu
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Guoyin Yin
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorYang Bao
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorTong Yao
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorWeiyu Kong
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorYangyang Li
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Ying Fu
College of Arts and Sciences, Northeast Agricultural University, Harbin, Heilongjiang, 150030 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Dong Wu
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Guoyin Yin
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorDedicated to the Special Issue of Boron Chemistry.
Comprehensive Summary
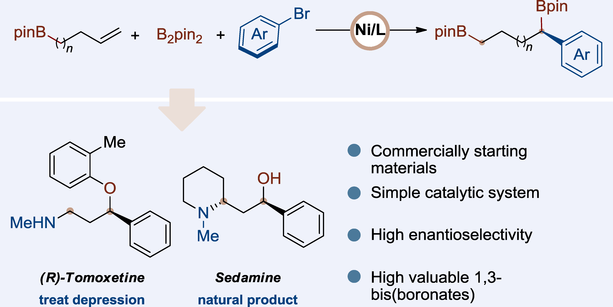
Chiral 1,3-substituted fragments are ubiquitous in pharmaceutical molecules and natural products, prompting the development of numerous methods to access these structures. Nonetheless, devising synthetic routes for complex chiral architectures in practical applications typically demands years of expertise. Herein, we developed a nickel-catalyzed enantioselective migratory arylboration reaction of allylboronic esters using a chiral 1,2-diamine ligand, yielding a range of chiral 1,3-bis(boronates) with high enantioselectivity. The protocol is characterized by its use of commercially available substrates, mild reaction conditions, user-friendly procedures, and a broad substrate compatibility. Moreover, we demonstrate the practicality and application potential of this reaction by synthesizing several key drug intermediates.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400742-sup-0001-supinfo.pdfPDF document, 7.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Huang, Y.; Gao, J.; Gao, P.; Peng, D.; Dai, Y.; Jiang, H.; Zhang, X. A comprehensive assessment of genetic variation in serotonin transporter gene (5-HTTLPR+ rs25531) and the response to dapoxetine in Chinese patients with premature ejaculation. Andrologia 2021, 53, e14141.
- 2 Schneider, M. P.; Goergens, U. An efficient route to enantiomerically pure antidepressants: tomoxetine, nisoxetine and fluoxetine. Tetrahedron : Asymmetry 1992, 3, 525–528.
- 3
Yadav, J.; Reddy, M. S.; Rao, P. P.; Prasad, A. Enantioselective synthesis of (+)-sedamine and (-)-allosedamine. Synthesis 2006, 2006, 4005–4012.
10.1055/s-2006-950331 Google Scholar
- 4(a) Nicolaou, K.; Sorensen, E.; Winssinger, N. The art and science of organic and natural products synthesis. J. Chem. Educ. 1998, 75, 1225;
(b) Nicolaou, K.; Vourloumis, D.; Winssinger, N.; Baran, P. S. The art and science of total synthesis at the dawn of the twenty-first century. Angew. Chem. Int. Ed. 2000, 39, 44–122.
10.1002/(SICI)1521-3773(20000103)39:1<44::AID-ANIE44>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 5(a) Harwood, S. J.; Palkowitz, M. D.; Gannett, C. N.; Perez, P.; Yao, Z.; Sun, L.; Abruña, H. D.; Anderson, S. L.; Baran, P. S. Modular terpene synthesis enabled by mild electrochemical couplings. Science 2022, 375, 745–752; (b) Seebach, D. Methods of reactivity umpolung. Angew. Chem. Int. Ed. 1979, 18, 239–258; (c) Zhang, B.; Gao, Y.; Hioki, Y.; Oderinde, M. S.; Qiao, J. X.; Rodriguez, K. X.; Zhang, H.-J.; Kawamata, Y.; Baran, P. S. Ni-electrocatalytic C sp3–C sp3 doubly decarboxylative coupling. Nature 2022, 606, 313–318.
- 6 Kurti, L.; Czakó, B. Strategic Applications of Named Reactions in Organic Synthesis, Elsevier, 2005.
- 7(a) Gao, Y.; Klunder, J. M.; Hanson, R. M.; Masamune, H.; Ko, S. Y.; Sharpless, K. B. Catalytic asymmetric epoxidation and kinetic resolution: modified procedures including in situ derivatization. J. Am. Chem. Soc. 1987, 109, 5765–5780; (b) Sunazuka, T.; Hirose, T.; Shirahata, T.; Harigaya, Y.; Hayashi, M.; Komiyama, K.; Ōmura, S.; Smith, A. B. Total synthesis of (+)-madindoline A and (−)-madindoline B, potent, selective inhibitors of interleukin 6. Determination of the relative and absolute configurations. J. Am. Chem. Soc. 2000, 122, 2122–2123; (c) Volchkov, I.; Lee, D. Asymmetric total synthesis of (−)-amphidinolide V through effective combinations of catalytic transformations. J. Am. Chem. Soc. 2013, 135, 5324–5327.
- 8(a) Warren, S.; Wyatt, P. Organic Synthesis: The Disconnection Approach, John Wiley & Sons, 2008;
(b) Hoffmann, R. W. Elements of Synthesis Planning, Vol. 307, Springer, 2009.
10.1007/978-3-540-79220-8 Google Scholar
- 9(a) Brooks, W. L.; Sumerlin, B. S. Synthesis and applications of boronic acid-containing polymers: from materials to medicine. Chem. Rev. 2016, 116, 1375–1397; (b) Diner, C.; Szabo, K. J. Recent advances in the preparation and application of allylboron species in organic synthesis. J. Am. Chem. Soc. 2017, 139, 2–14; (c) Kalita, S. J.; Cheng, F.; Huang, Y. Y. Recent Advances of Applying Boron-Reagents in Asymmetric Total Syntheses of Natural Products and Bio-Active Molecules. Adv. Synth. Catal. 2020, 362, 2778–2800; (d) Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483; (e) Rygus, J. P.; Crudden, C. M. Enantiospecific and iterative Suzuki–Miyaura cross-couplings. J. Am. Chem. Soc. 2017, 139, 18124–18137; (f) Trippier, P. C.; McGuigan, C. Boronic acids in medicinal chemistry: anticancer, antibacterial and antiviral applications. Med. Chem. Commun. 2010, 1, 183–198.
- 10(a) Crudden, C. M.; Glasspoole, B. W.; Lata, C. J. Expanding the scope of transformations of organoboron species: carbon–carbon bond formation with retention of configuration. Chem. Commun. 2009, 6704–6716; (b) Jana, R.; Pathak, T. P.; Sigman, M. S. Advances in transition metal (Pd, Ni, Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 2011, 111, 1417–1492; (c) Sandford, C.; Aggarwal, V. K. Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun. 2017, 53, 5481–5494.
- 11(a) Viso, A.; Fernández De La Pradilla, R.; Tortosa, M. Site-Selective Functionalization of C (sp3) Vicinal Boronic Esters. ACS Catal. 2022, 12, 10603–10620; (b) Wang, X.; Wang, Y.; Huang, W.; Xia, C.; Wu, L. Direct synthesis of multi (boronate) esters from alkenes and alkynes via hydroboration and boration reactions. ACS Catal. 2020, 11, 1–18; (c) Chen, C.; Wang, H.; Li, T.; Lu, D.; Li, J.; Zhang, X.; Hong, X.; Lu, Z. Cobalt-catalyzed asymmetric sequential hydroboration/isomerization/hydroboration of 2-arylVinylcyclopropanes. Angew. Chem. Int. Ed. 2022, 61, e202205619.
- 12(a) Feng, X.; Jeon, H.; Yun, J. Regio-and enantioselective copper (I)-catalyzed hydroboration of borylalkenes: asymmetric synthesis of 1,1-diborylalkanes. Angew. Chem. Int. Ed. 2013, 52, 3989–3992;
(b) Lee, J. C. H.; McDonald, R.; Hall, D. G. Enantioselective preparation and chemoselective cross-coupling of 1,1-diboron compounds. Nat. Chem. 2011, 3, 894–899;
(c) Mlynarski, S. N.; Schuster, C. H.; Morken, J. P. Asymmetric synthesis from terminal alkenes by cascades of diboration and cross-coupling. Nature 2014, 505, 386–390;
(d) Wiesauer, C.; Weissensteiner, W. Rhodium catalysed enantioselective hydroboration of alkenylboronic esters with catecholborane. Tetrahedron:
Asymmetry 1996, 7, 5–8;
(e) Blair, D. J.; Tanini, D.; Bateman, J. M.; Scott, H. K.; Myers, E. L.; Aggarwal, V. K. Selective uni-and bidirectional homologation of diborylmethane. Chem. Sci. 2017, 8, 2898–2903;
(f) Fawcett, A.; Nitsch, D.; Ali, M.; Bateman, J. M.; Myers, E. L.; Aggarwal, V. K. Regio-and Stereoselective Homologation of 1, 2-Bis (Boronic Esters): Stereocontrolled Synthesis of 1,3-Diols and Sch 725674. Angew. Chem. Int. Ed. 2016, 128, 14883–14887;
10.1002/ange.201608406 Google Scholar(g) Schuster, C. H.; Li, B.; Morken, J. P. Modular Monodentate Oxaphospholane (OxaPhos) Ligands: Utility in Highly Efficient and Enantioselective 1,4-Diboration of 1,3-Dienes. Angew. Chem. Int. Ed. 2011, 50, 7906–7909.
- 13(a) Li, Y.; Li, Y.; Shi, H.; Wei, H.; Li, H.; Funes-Ardoiz, I.; Yin, G. Modular access to substituted cyclohexanes with kinetic stereocontrol. Science 2022, 376, 749–753; (b) Wu, D.; Kong, W.; Bao, Y.; Zhao, D.; Li, Y.; Yin, G. Alkene 1,1-difunctionalizations via organometallic-radical relay. Nat. Catal. 2023, 6, 1030–1041; (c) Wang, W.; Ding, C.; Yin, G. Catalyst-controlled enantioselective 1,1-arylboration of unactivated olefins. Nat. Catal. 2020, 3, 951–958; (d) Shen, Z.; Yin, G.; Li, Y. Stereoselective Synthesis of 2-Deoxy-α-N-Glycosides from Glycals with 1,4,2-Dioxazol-5-ones. Chin. J. Chem. 2024, 42, 2147–2152; (e) Ren, Y.; Wang, L.; Ding, C.; Li, Y.; Yin, G. Nickel-Catalyzed Stereoselective Migratory Carboboration of 1, 4-Cyclohexadiene. Chin. J. Chem. 2024, 42, 356–362; (f) Wu, D.; Pang, H.; Yin, G. 1,1-Regioselective alkenylboration of styrenes enabled by palladium catalysis. Chin. Chem. Lett. 2023, 34, 108087–108091; (g) Sun, C.; Yin, G. Integrating aryl chlorides into nickel-catalyzed 1, 1-difunctionalization of alkenes. Chin. Chem. Lett. 2022, 33, 5096–5100.
- 14(a) Zhang, M.; Ji, Y.; Zhang, C. Transition metal catalyzed enantioselective migratory function-alization reactions of alkenes through chain-walking. Chin. J. Chem. 2022, 40, 1608–1622;
(b) Guo, Q.; Shen, X.; Lu, Z. Recent advances in enantioselective reactions of terminal unactivated alkenes. Chin. J. Chem. 2024, 42, 760–776;
(c) Sun, C.; Li, Y.; Yin, G. Practical Synthesis of Chiral Allylboronates by Asymmetric 1,1-Difunctionalization of Terminal Alkenes. Angew. Chem. Int. Ed. 2022, 134, e202209076.
10.1002/ange.202209076 Google Scholar
- 15 Liang, Y.; Narayanasamy, J.; Schinazi, R. F.; Chu, C. K. Phosphoramidate and phosphate prodrugs of (−)-β-d-(2R,4R)-dioxolane-thymine: Synthesis, anti-HIV activity and stability studies. Bioorg. Med. Chem. 2006, 14, 2178–2189.
- 16(a) Yan, L.; Morken, J. P. Site-Selective Mono-Oxidation of 1,2-Bis (boronates). Org. Lett. 2019, 21, 3760–3763; (b) Zhang, M.; Liu, Z.; Zhao, W. Rhodium-Catalyzed Remote Borylation of Alkynes and Vinylboronates. Angew. Chem. Int. Ed. 2023, 62, e202215455.
- 17(a) Imao, D.; Glasspoole, B. W.; Laberge, V. S.; Crudden, C. M. Cross coupling reactions of chiral secondary organoboronic esters with retention of configuration. J. Am. Chem. Soc. 2009, 131, 5024–5025; (b) Sun, C.; Potter, B.; Morken, J. P. A catalytic enantiotopic-group-selective Suzuki reaction for the construction of chiral organoboronates. J. Am. Chem. Soc. 2014, 136, 6534–6537.
- 18(a) Kayano, K.; Tsutsumi, T.; Murata, Y.; Ogasa, C.; Watanabe, T.; Sato, R.; Karanjit, S.; Namba, K. Epoxide Ring-Opening Reactions for Abundant Production of Mugineic Acids and Nicotianamine Probes. Angew. Chem. Int. Ed. 2024, 63, e202401411; (b) Matsuura, F.; Hamada, Y.; Shioiri, T. Total synthesis of mugineic acid. Efficient use of the phenyl group as the carboxyl synthon. Tetrahedron 1993, 49, 8211–8222.
- 19(a) Kamal, A.; Khanna, G. R.; Ramu, R. Chemoenzymatic synthesis of both enantiomers of fluoxetine, tomoxetine and nisoxetine: lipase- catalyzed resolution of 3-aryl-3-hydroxypropanenitriles. Tetrahedron: Asymmetry 2002, 13, 2039–2051; (b) Xu, C.; Yuan, C. Candida Rugosa lipase-catalyzed kinetic resolution of β-hydroxy-β-arylpropionates and δ-hydroxy-δ-aryl-β-oxo-pentanoates. Tetrahedron 2005, 61, 2169–2186.
- 20(a) Kato, Y.; Scheuer, P. J. Aplysiatoxin and debromoaplysiatoxin, constituents of the marine mollusk Stylocheilus longicauda. J. Am. Chem. Soc. 1974, 96, 2245–2246; (b) Kato, Y.; Scheuer, P. The aplysiatoxins. Pure Appl. Chem. 1975, 41, 1–14; (c) Moore, R. E.; Blackman, A. J.; Cheuk, C. E.; Mynderse, J. S.; Matsumoto, G. K.; Clardy, J.; Woodard, R. W.; Craig, J. C. Absolute stereochemistries of the aplysiatoxins and oscillatoxin A. J. Org. Chem. 1984, 49, 2484–2489; (d) Okamura, H.; Kuroda, S.; Ikegami, S.; Tomita, K.; Sugimoto, Y.-i.; Sakaguchi, S.-i.; Ito, Y.; Katsuki, T.; Yamaguchi, M. A formal synthesis of aplysiatoxin: enantioselective synthesis of Kishi's aldehyde. Tetrahedron 1993, 49, 10531–10554.
- 21 Reddy, K. R.; Matelich, M. C.; Ugarkar, B. G.; Gómez-Galeno, J. E.; DaRe, J.; Ollis, K.; Sun, Z.; Craigo, W.; Colby, T. J.; Fujitaki, J. M.; Boyer, S. H.; Poelje, P. D. v.; Erion, M. D. Pradefovir: a prodrug that targets adefovir to the liver for the treatment of hepatitis B. J. Med. Chem. 2008, 51, 666–676.
- 22 Choi, E. T.; Lee, M. H.; Kim, Y.; Park, Y. S. Asymmetric dehydration of β-hydroxy esters and application to the syntheses of flavane derivatives. Tetrahedron 2008, 64, 1515–1522.




