Recent Advances in the Development of Indolizine Scaffolds: Synthetic Methodology and Mechanistic Insights
Corresponding Author
Jiuzhong Huang
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
These authors contributed equally to the present work.
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorChunsheng Li
School of Chemistry and Chemical Engineering, Zhaoqing University, Zhaoqing, Guangdong, 526060 China
These authors contributed equally to the present work.
Search for more papers by this authorXiaoning Li
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
These authors contributed equally to the present work.
Search for more papers by this authorGuangyu Li
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorZiyi Yang
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorYinke Yu
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorYanping Xie
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorCorresponding Author
Hao Huang
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Fuchao Yu
Faculty of Life Science and Technology, Kunming University of Science and Technology, Kunming, Yunnan, 650500 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Zhihua He
First Affiliated Hospital, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jiuzhong Huang
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
These authors contributed equally to the present work.
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorChunsheng Li
School of Chemistry and Chemical Engineering, Zhaoqing University, Zhaoqing, Guangdong, 526060 China
These authors contributed equally to the present work.
Search for more papers by this authorXiaoning Li
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
These authors contributed equally to the present work.
Search for more papers by this authorGuangyu Li
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorZiyi Yang
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorYinke Yu
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorYanping Xie
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorCorresponding Author
Hao Huang
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Fuchao Yu
Faculty of Life Science and Technology, Kunming University of Science and Technology, Kunming, Yunnan, 650500 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Zhihua He
First Affiliated Hospital, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorAbstract
Comprehensive Summary
Indolizine is a nitrogen-containing heterocycle with strong aromaticity, possessing a delocalized 10π-electron system. Based on the indolizine scaffolds, numerous molecules with biological activity and organic functional materials have been synthesized. Since 2016, over 110 papers have been published on the synthesis of indolizine scaffolds, but the reviews on synthesizing indolizine scaffolds have been incomplete and not up-to-date. Herein, from the perspective of the structure of indolizine with the combination of pyrrole and pyridine ring, we focus on the construction of indolizine scaffolds through the diversity of starting substrates, including pyridine derivatives (N1-substituted pyridinium salt derivatives, C2-substituted pyridine derivatives, N1- and C2-free substituted pyridine derivatives), pyrrole derivatives and unoriginal ring substrates. Furthermore, the corresponding reaction mechanisms of synthetic methodologies are also elaborated. Therefore, this review not only paves the way for indolizine synthesis but also provides insight into exploring new reaction modes for constructing nitrogen-containing heterocycles.

Key Scientists
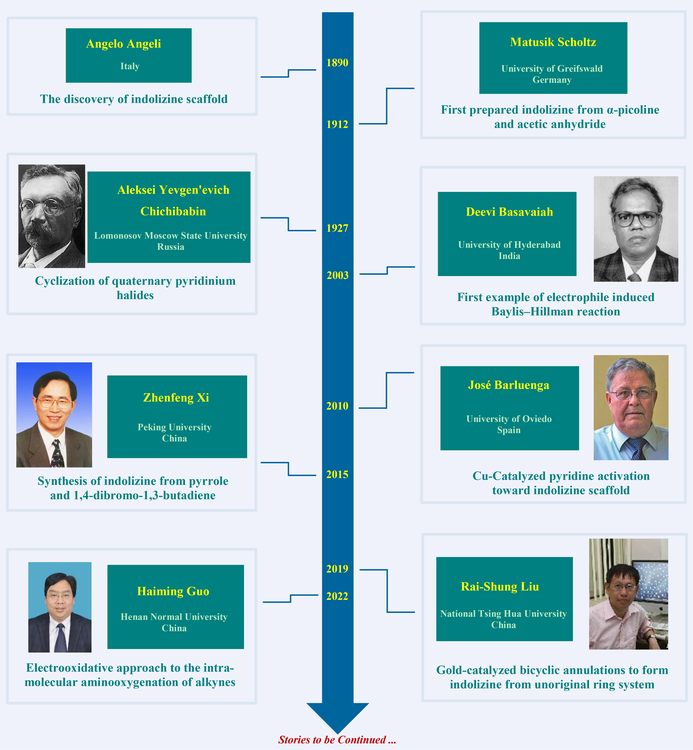
Indolizine was discovered by Angeli in 1890 and first prepared by Scholtz in 1912 from α-picoline and acetic anhydride. A general approach was developed by Chichibabin in 1927, that is of practical value for the preparation of 2-alkyl- or 2-arylindolizines. The Chichibabin reaction was the ring closure of quaternary pyridinium halides. At the begining of the 21st century, Basavaiah introduced a new dimension in the Baylis-Hillman chemistry leading to a novel facile convenient methodology for synthesis of indolizine scaffolds in one-pot operation. In 2010, Barluenga reported Cu(I)-catalyzed regioselective [3+2] cyclization of unsubstituted pyridines toward alkenyldiazoacetates leading to functionalized indolizine derivatives, that was the first successful example of metal-catalyzed cyclization of a π-deficient heterocyclic system with alkenyldiazo compounds. In 2019 and 2022, Xi and Liu exploited the methods of non-pyridine derivatives as starting materials to synthesize indolizines, respectively. In 2022, Guo developed an environmentally benign electrooxidative approach for constructing formyl- and acyl-substituted indolizines.
References
- 1 Shigemitsu, Y.; Komiya, K.; Mizuyama, N.; Tominaga, Y. TD-DFT investigation on the electronic spectra of novel N-methylmaleimides linked with indolizine ring system. J. Mol. Struct.: THEOCHEM 2008, 855, 92–101.
- 2
Park, C. H.; Ryabova, V.; Seregin, I. V.; Sromek, A. W.; Gevorgyan, V. Palladium-catalyzed arylation and heteroarylation of indolizines. Org. Lett. 2008, 6, 1159–1162.
10.1021/ol049866q Google Scholar
- 3 Li, Y.; Hu, H. Y.; Ye, J. P.; Fun, H. K.; Hu, H. W.; Xu, J. H. Reaction modes and mechanism in indolizine photooxygenation reactions. J. Org. Chem. 2004, 69, 2332–2339.
- 4 George, M. V.; Bhat, V. Photooxygenations of nitrogen heterocycles. Chem. Rev. 1979, 79, 447–478.
- 5 Woodward, R. B.; Cava, M. P.; Ollis, W. D.; Hunger, A.; Daeniker, H. U.; Schenker, K. The Total Synthesis of Strychnine. Tetrahedron 1963, 19, 247–288.
- 6 Li, C.; Blackman, A. J. Perhydropyrrolo[2,1-j]quinolin-7-one Alkaloids from the Ascidian Clavelina Cylindrica. Aust. J. Chem. 1994, 47, 1355–1361.
- 7 Joule, J. A.; Mills, K. Heterocyclic Chemistry, Wiley, 2010.
- 8 Daly, J. W.; Spande, T. F.; Garraffo, H. M. Alkaloids from Amphibian Skin: A Tabulation of Over Eight-Hundred Compounds. J. Nat. Prod. 2005, 68, 1556–1575.
- 9 Stephens, P. J.; Pan, J. J.; Devlin, F. J.; Krohn, K.; Kurtán, T. Determination of the absolute configurations of natural products via density functional theory calculations of vibrational circular dichroism, electronic circular dichroism, and optical rotation: the iridoids plumericin and isoplumericin. J. Org. Chem. 2007, 72, 3521–3536.
- 10 Saporito, R. A.; Donnelly, M. A.; Jain, P.; Garraffo, H. M.; Spande, T. F.; Daly, J. W. Spatial and temporal patterns of alkaloid variation in the poison frog Oophaga pumilio in Costa Rica and Panama over 30 years. Toxicon 2007, 50, 757–778.
- 11 Taylor, R. D.; MacCoss, M.; Lawson, A. D. G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859.
- 12 Michael, J. P. Indolizidine and quinolizidine alkaloids. Nat. Prod. Rep. 2001, 18, 520–542.
- 13 Michael, J. P. Indolizidine and quinolizidine alkaloids. Nat. Prod. Rep. 2002, 19, 719–741.
- 14 Michael, J. P. Indolizidine and quinolizidine alkaloids. Nat. Prod. Rep. 2008, 25, 139–165.
- 15 Xiang, Q.; Wang, C.; Wu, T.; Zhang, C.; Hu, Q.; Luo, G.; Hu, J.; Zhuang, X.; Zou, L.; Shen, H.; Wu, X.; Zhang, Y.; Kong, X.; Liu, J.; Xu, Y. Design, synthesis, and biological eval-uation of 1-(indolizin-3-yl)ethan-1-ones as CBP bromodomain inhibitors for the treatment of prostate cancer. J. Med. Chem. 2022, 65, 785–810.
- 16 Albaladejo, M. J.; González-Soria, M. J.; Alonso, F. Metal-free remote-site C-H alkenylation: regio- and diastereoselective synthesis of solvatochromic dyes. Green Chem. 2018, 20, 701–712.
- 17 Moon, S. H.; Jung, Y.; Kim, S. H.; Kim, I. Synthesis, characterization and biological evaluation of anti-cancer indolizine derivatives via inhibiting β-catenin activity and activating p53. Bioorg. Med. Chem. Lett. 2016, 26, 110–113.
- 18 Sharma, V.; Kumar, V. Indolizine: a biologically active moiety. Med. Chem. Res. 2014, 23, 3593–3606.
- 19 Wan, J.; Zheng, C. J.; Fung, M. K.; Liu, X. K.; Lee, C. S.; Zhang, X. H. Multifunctional electron-transporting in-dolizine derivatives for highly efficient blue fluorescence, orange phosphorescence host and two-color based white OLEDs. J. Mater. Chem. 2012, 22, 4502–4510.
- 20
Flitsch, W. Pyrroles with Fused Six-Membered Heterocyclic Rings: A-Fused, Pergamon Press, Oxford, UK, 1984.
10.1016/B978-008096519-2.00058-8 Google Scholar
- 21 Wu, G.; Xu, X.; Wang, S.; Chen, L.; Pang, B.; Ma, T.; Ji, Y. Chin. Chem. Lett. 2022, 33, 2005–2008.
- 22 Neto, J. S. S.; Gilson Zeni, G. Recent developments in the cyclization of alkynes and nitrogen compounds for the synthesis of indole derivatives. Asian J. Org. Chem. 2021, 10, 1282–1318.
- 23 Joshi, D. R.; Ikyon Kim, I Recent advances in the synthesis of N-fused heterocycles from N-aroylmethylpyrrole-2-carboxaldehyde derivatives via annulative functionalization strategies. Tetrahedron 2024, 151, 133787–133796.
- 24 Basavaiah, D.; Rao, A. J. First example of electrophile induced Baylis–Hillman reaction: a novel facile one-pot synthesis of indolizine derivatives. Chem. Commun. 2003, 604–605.
- 25 Barluenga, J.; Lonzi, G.; Riesgo, L.; López, L. A.; Miguel Tomás, M. Pyridine activation via copper(I)-catalyzed annulation toward indolizines. J. Am. Chem. Soc. 2010, 132, 13200–13202.
- 26 Hao, W.; Wang, H.; Ye, Q.; Zhang, W.-X.; Xi, Z. Cyclopentadiene- Phosphine/palladium-catalyzed synthesis of indolizines from pyrrole and 1,4-dibromo-1,3-butadienes. Org. Lett. 2015, 17, 5674–5677.
- 27 Čerņaks, D. Recent methods for the synthesis of indolizines. Chem. Heterocycl. Compd. 2016, 52, 524–526.
- 28 Sadowski, B.; Klajn, J.; Gryko, D. T. Recent advances in the synthesis of indolizines and their π-expanded analogues. Org. Biomol. Chem. 2016, 14, 7804–7828.
- 29 Nevskaya, A.; Zinoveva, A. D.; Van der Eycken, E. V.; Voskressensky, L. G. Synthetic Strategies for the Construction of Indolizines and Indolizine-fused Compounds: Thienoindolizines and Indolizinoindoles. Asian J. Org. Chem. 2023, 12, e202300359.
- 30 Hui, J.; Ma, Y.; Zhao, J.; Cao, H. Recent advances in the synthesis of indolizine and its derivatives by radical cyclization/cross-coupling. Org. Biomol. Chem. 2021, 19, 10245–10258.
- 31The search result provided by CAS SciFindern in May 10, 2024.
- 32 Escolano, M.; Gaviña, D.; Alzuet-Piña, G.; Díaz-Oltra, S.; Sánchez-Roselló, M.; Pozo, C. Recent Strategies in the Nucleophilic Dearomatization of Pyridines, Quinolines, and Isoquinolines. Chem. Rev. 2024, 124, 1122–1246.
- 33 Thakur, A.; Louie, J. Advances in nickel-catalyzed cycloaddition reactions to construct carbocycles and heterocycles. Acc. Chem. Res. 2015, 48, 2354–2365.
- 34 Bonte, S.; Ghinea, I. O.; Dinica, R.; Baussanne, I.; Demeunynck, M. Investigation of the Pyridinium Ylide-Alkyne Cycloaddition as a Fluorogenic Coupling Reaction. Molecules 2016, 21, 332–347.
- 35 Dohmen, C.; Ihmels, H.; Kreienmeier, R.; Patrick, B. O. Synthesis of a Crystallochromic Indolizine Dye by a Base-and Catalyst-Free Photochemical Route. Chem. Commun. 2019, 55, 11071–11074.
- 36 Moise, M.; Ghinet, A.; Shova, S.; Bîcu, E. Switching the Reactivity of Cyanomethylpyridinium Salts in the 1,3-cycloaddition Conditions with Alkyl Propiolates to Cyanoindolizines or Cyanoazaindolizinyl- Indolizines. Tetrahedron 2020, 76, 131502.
- 37 Zhao, L.; Li, W.; Liu, J.; Ni, L.; Liu, Z.; Shen, H.; Cao, H.; Liu, X. Transition Metal-Free Annulative Vinylene Transfer via the 1, 3-Dipolar Reaction of N-ylides: Access to Benzo-Fused Indolizines. Org. Biomol. Chem. 2022, 20, 9604–9608.
- 38 Penteado, F.; Gomes, C. S.; Perin, G.; Garcia, C. S.; Bortolatto, C. F.; Brüning, C. A.; Lenardão, E. J. Regioselective Synthesis of 1-Sulfanyl- and 1-Selanylindolizines. J. Org. Chem. 2019, 84, 7189–7198.
- 39 Hou, X.; Zhou, S.; Li, Y.; Guo, M.; Zhao, W.; Tang, X.; Wang, G. Synthesis of Indolizines from Pyridinium Salts and Ethyl Bromodifluoroacetate. Org. Lett. 2020, 22, 9313–9318.
- 40 Yang, L. M.; Zhang, Y. Y.; Deng, J. T.; Ma, A. J.; Zhang, X. Z.; Zhang, S. Y.; Peng, J. B. Oxidative [3+2] Annulation of Pyridinium Salts with gem-Difluoroalkenes: Synthesis of 2-Fluoroindolizines. Asian J. Org. Chem. 2021, 10, 1679–1682.
- 41 Zhang, Q.; Hu, D.; Song, J.; Ren, H. [3+2]-Annulation of gem-Difluoroalkenes and Pyridinium Ylides: Access to Functionalized 2-Fluoroindolizines. J. Org. Chem. 2021, 86, 4646–4660.
- 42 Jadala, C.; Reddy, V. G.; Krishna, N. H.; Shankaraiah, N.; Kamal, A. Base-Mediated 1,3-Dipolar Cycloaddition of Pyridinium Bromides with Bromoallyl Sulfones: A Facile Access to Indolizine Scaffolds. Org. Biomol. Chem. 2020, 18, 8694–8701.
- 43 Cheng, B.; Zhang, X.; Li, Y.; Li, H.; He, Y.; Li, Y.; Zhai, H. Synthesis of Indolizines from Pyridinium 1, 4-Zwitterionic Thiolates and α-Functionalized Bromoalkanes via a Stepwise [(5+1)-1] Pathway. Chem. Commun. 2020, 56, 8396–8399.
- 44 Cheng, B.; Li, Y.; Zhang, X.; Duan, S.; Li, H.; He, Y.; Li, Y.; Wang, T.; Zhai, H. Two Reaction Modes of Pyridinium 1,4-Zwitterionic Thiolates with Sulfenes: Synthesis of 3H-1,2-Dithiole 2,2-Dioxides, 1,9a-Dihydropyrido[2,1-c][1,4]thiazines, and Indolizines. Org. Lett. 2020, 22, 5817–5821.
- 45 Cheng, B.; Li, H.; Duan, S.; Zhang, X.; He, Y.; Li, Y.; Li, Y.; Wang, T.; Zhai, H. Synthesis of Indolizines from Pyridinium 1,4-Zwitterionic Thiolates and Propiolic Acid Derivatives via a Formal [4+1] Pathway. Org. Biomol. Chem. 2020, 18, 6253–6257.
- 46 Monreal-Corona, R.; Díaz-Jiménez, À.; Roglans, A.; Poater, A.; Pla-Quintana, A. Indolizine Synthesis through Annulation of Pyridinium 1,4-Thiolates and Copper Carbenes: A Predictive Catalysis Approach. Adv. Synth. Catal. 2023, 365, 760–766.
- 47 Li, W.; Wang, H.; Zhang, Y.; Zhao, L.; Guo, P.; Cao, H.; Liu, X. Copper- Catalyzed Formal [4+1] Annulation toward Diverse Trifunctionalized Indolizines from Pyridinium 1,4-Zwitterionic Thiolates and Diazos. J. Org. Chem. 2023, 88, 7199–7207.
- 48 Sun, S.; Wei, Y.; Xu, J. Microwave-Mediated Stereocontrolled Annulations of Diazo (aryl)methyl(diaryl)phosphine Oxides with Pyridinium 1,4-Zwitterionic Thiolates. Chem. Commun. 2023, 59, 239–242.
- 49 Jin, S.; Wang, L.; Han, H.; Liu, X.; Bu, Z.; Wang, Q. Assembly of Functionalized π-Extended Indolizine Polycycles through Dearomative [3+2] Cycloaddition/Oxidative Decarbonylation. Chem. Commun. 2021, 57, 359–362.
- 50 Aksenov, V.; Arutiunov, N. A.; Kirilov, N. K.; Aksenov, D. A.; Grishin, I. Y.; Aksenov, N. A.; Wang, H.; Du, L.; Betancourt, T.; Pelly, S. C.; Kornienko, A.; Rubin, M. [3+2]-Annulation of Pyridinium Ylides with 1-Chloro-2-Nitrostyrenes Unveils a Tubulin Polymerization Inhibitor. Org. Biomol. Chem. 2021, 19, 7234–7245.
- 51 Shen, B.; Li, B.; Wang, B. Rh(III)-Catalyzed Oxidative Annulation Leading to Substituted Indolizines by Cleavage of C(sp2)-H/C(sp3)-H Bonds. Org. Lett. 2016, 18, 2816–2819.
- 52 Li, K.; Li, C. Enantioselective Synthesis of 3-Allylindolizines via Sequential Rh-Catalyzed Asymmetric Allylation and Tschitschibabin. Org. Lett. 2020, 22, 9456–9461.
- 53 Gou, Q.; Zhu, Q.; Deng, M.; Li, W.; Ran, X.; Xie, J.; Huang, H.; Tan, X.; Zhu, M. The Regioselective Annulation of N-Methylpyridinium Ylides with Alkenes Enabled by Palladium Catalysis: Access to 3-Unsubstituted Indolizine Derivatives. Org. Chem. Front. 2022, 9, 4719–4725.
- 54 Chen, Y.; Shatskiy, A.; Liu, J. Q.; Kärkäs, M. D.; Wang, X. S. Silver- Promoted (4+1) Annulation of Isocyanoacetates with Alkylpyridinium Salts: Divergent Regioselective Synthesis of 1, 2-Disubstituted Indolizines. Org. Lett. 2021, 23, 7555–7560.
- 55 Zhang, D.; Su, Z.; He, Q.; Wu, Z.; Zhou, Y.; Pan, C.; Liu, X.; Feng, X. Diversified Transformations of Tetrahydroindolizines to Construct Chiral 3-Arylindolizines and Dicarbofunctionalized 1,5-Diketones. J. Am. Chem. Soc. 2020, 142, 15975–15985.
- 56 Miao, C. B.; Qiang, X. Q.; Xu, X.; Song, X. Q.; Zhou, S. Q.; Lyu, X.; Yang, H. T. Synthesis of Stable N-H Imines with a Benzo[7,8]indolizine Core and Benzo[7,8]indolizino[1,2-c]quinolines via Copper-Catalyzed Annulation of α,β-Unsaturated O-Acyl Ketoximes with Isoquinolinium N-Ylides. Org. Lett. 2022, 24, 3828–3833.
- 57 Yang, G.; Li, Z.; Liu, Y.; Guo, D.; Sheng, X.; Wang, J. Organocatalytic Higher-Order [8+2] Cycloaddition for the Assembly of Atropoenantiomeric 3-Arylindolizines. Org. Lett. 2021, 23, 8109–8113.
- 58 Yang, W. W.; Ye, Y. F.; Chen, L. L.; Fu, J. Y.; Zhu, J. Y.; Wang, Y. B. Catalyst-and Additive-Free Annulation of Ynediones and (Iso) Quinoline N-Oxides: An Approach to Synthesis of Pyrrolo [2, 1-a] Isoquinolines and Pyrrolo [1, 2-a] Quinolines. J. Org. Chem. 2020, 86, 169–177.
- 59 Shu, W. M.; He, J. X.; Zhang, X. F.; Wang, S.; Wu, A. X. TFA-Mediated DMSO-Participant Sequential Oxidation/1,3-Dipolar Cycloaddition Cascade of Pyridinium Ylides for the Assembly of Indolizines. J. Org. Chem. 2019, 84, 2962–2968.
- 60 Shi, F.; Zhang, Y.; Lu, Z.; Zhu, X.; Kan, W.; Wang, X.; Hu, H. Transition- Metal-Free Synthesis of Indolizines from Electron-Deficient Alkenes via One-Pot Reaction Using TEMPO as an Oxidant. Synthesis 2016, 48, 413–420.
- 61 Liu, Y.; Hu, H.; Zhou, J.; Wang, W.; He, Y.; Wang, C. Application of Primary Halogenated Hydrocarbons for the Synthesis of 3-Aryl and 3-Alkyl Indolizines. Org. Biomol. Chem. 2017, 15, 5016–5024.
- 62 Zhang, X.; Lu, G.; Xu, Z.; Cai, C. Facile Synthesis of Indolizines via 1,3-Dipolar Cycloadditions in [Omim]Br: The Promotion of the Reaction through Noncovalent Interactions. ACS Sustainable Chem. Eng. 2017, 5, 9279–9285.
- 63 Pan, D.; Liu, F. X.; Zeng, Z.; Ye, J.; Cai, Y.; Wang, S.; Zhou, Z.; Yi, W. Practical Conversion of Gem-Difluorocyclopropenes for the Chemodivergent Assembly of Fluorinated Heterocyclic Frameworks. Green Chem. 2023, 25, 10630–10637.
- 64 Wu, C.-Y.; Chen, X.-L.; Yang, D.-S.; Tang, Y.-X.; Wang, L.-S.; Wu, Y.-D.; Zhuang, S.-Y.; Wu, A.-X. Base-controlled selective cleavage of the C-F bond of difluorocarbene for the divergent assembly of indolizines. Org. Chem. Front. 2024, 11, 4214–4218.
- 65 Oh, H.; Kim, S. M.; Park, S. Y.; Park, J. K. Base-Controlled Cu-Catalyzed Tandem Cyclization/Alkynylation for the Synthesis of Indolizines. Org. Lett. 2016, 18, 2204–2207.
- 66 Goulart, T. A. C.; Back, D. F.; Zeni, G. Copper-Catalyzed Carbon-Nitrogen/Carbon-Selenium Bonds Formation: Synthesis of 2-(Organochalcogenyl)-Indolizines. Adv. Synth. Catal. 2017, 359, 1901–1911.
- 67 Li, Y.; Xiong, W.; Zhang, Z.; Xu, T. Synthesis of Indolizine Derivatives Triggered by the Oxidative Addition of Aroyl Chloride to Pd(0) Complex. J. Org. Chem. 2020, 85, 6392–6399.
- 68 Xiao, X.; Han, P.; Zhou, H.; Liu, J. Palladium-Catalyzed Difunctionalization of Alkenes by Relay Coupling with Propargylic Pyridines: Synthesis of Indolizine and Indolizinone-Containing Bisheterocycles. J. Org. Chem. 2021, 86, 18179–18191
- 69 Bagle, P. N.; Mane, M. V.; Vanka, K.; Shinde, D. R.; Shaikh, S. R.; Gonnade, R. G.; Patil, N. T. Au(I)/Ag(I) Co-operative Catalysis: Interception of Ag-Bound Carbocations with α-Gold (I) enals in the Imino-Alkyne Cyclizations with N-Allenamides. Chem. Commun. 2016, 52, 14462–14465.
- 70 Chen, X.; Hu, X.; Deng, Y.; Jiang, H.; Zeng, W. A [4+1] Cyclative Capture Access to Indolizines via Cobalt(III)-Catalyzed Csp2-H Bond Functionalization. Org. Lett. 2016, 18, 4742–4745.
- 71 Gong, T.-J.; Xu, M.-Y.; Yu, S.-H.; Yu, C.-G.; Su, W.; Lu, X.; Xiao, B.; Fu, Y. Rhodium(III)-Catalyzed Directed C-H Coupling with Methyl Trifluoroacrylate: Diverse Synthesis of Fluoroalkenes and Heterocycles. Org. Lett. 2018, 20, 570–573.
- 72 Rossler, D.; Hartgerink, C. T.; Zerull, E. E.; Boss, B. L.; Frndak, A. K.; Mason, M. M.; Nickerson, L. A.; Romero, E. O.; Van de Burg, J. E.; Staples, R. J.; Anderson, C. E. Au(I)-Catalyzed Synthesis of Trisubstituted Indolizines from 2-Propargyloxypyridines and Methyl Ketones. Org. Lett. 2019, 21, 5591–5595.
- 73 Anderson, C. E.; Bos, H. I.; Dreher, D. M.; Hartgerink, C. T.; Scholtens, C. J.; Staples, R. J. Synthesis of Ester-Substituted Indolizines from 2-Propargyloxypyridines and 1,3-Dicarbonyls. J. Org. Chem. 2022, 87, 10241–10249.
- 74 Han, C.; Liu, Y.; Tian, X.; Rominger, F.; Hashmi, A. S. K. Dual, Gold/Silver Catalysis: Indolizines from 2-Substituted Pyridine Derivatives via a Tandem C(sp3)-H Alkynylation/Iminoauration. Org. Lett. 2021, 23, 9480–9484.
- 75 Lv, K. H.; Zhao, Q. S.; Zhao, K. H.; Yang, J. M.; Yan, S. J. Cu-Catalyzed Oxidative [3+2] Annulation of 2-(Pyridine-2-yl)acetates with Maleimides: Synthesis of 1H-Pyrrolo[3,4-b]indolizine-1,3-diones. J. Org. Chem. 2022, 87, 15301–15311.
- 76 Pathipati, S. R.; van der Werf, A.; Selander, N. Diastereoselective Synthesis of Polycyclic Indolizines with 2-(2-Enynyl)pyridines and Enamines. Org. Lett. 2018, 20, 3691–3694.
- 77 Lu, C.-J.; Yu, X.; Chen, Y.-T.; Song, Q.-B.; Wang, H. Indolizine Synthesis via Copper-Catalyzed Cyclization of Gem-Difluoroalkenes and 2-(Pyridin-2-yl) acetate Derivatives. Org. Chem. Front. 2020, 7, 2313–2318.
- 78 Sahoo, S. R.; Sarkar, D.; Henkel, F.; Reuter, H. Copper(I)-Catalyzed Synthesis of Functionalized Indolizinones from Substituted Pyridine Homologated Ynones. J. Org. Chem. 2020, 85, 902–911.
- 79 Paluru, D. K.; Mahesh, S.; Ahmad, F.; Anand, R. V. A Cascade, Synthesis of Hetero-arylated Triarylmethanes Through a Double 5-endo-dig Cyclization Sequence. Chem.-Asian J. 2019, 14, 4688–4695.
- 80 Wu, T.; Chen, M.; Yang, Y. Synthesis of Indolizines via Palladium Catalyzed Annulation of Propargyl Carbonates and 2-(Pyridin-2-yl)acetonitrile Derivatives. J. Org. Chem. 2017, 82, 11304–11309.
- 81 Kim, H.; Kim, S.; Kim, J.; Son, J. Y.; Baek, Y.; Um, K.; Lee, P. H. One-Pot Synthesis of Indolizines via Sequential Rhodium-Catalyzed [2+1]-Cyclopropanation, Palladium-Catalyzed Ring Expansion, and Oxidation Reactions from Pyridotriazoles and 1,3-Dienes. Org. Lett. 2017, 19, 5677–5680.
- 82 Joshi, D. R.; Kim, I. Regioselective Synthesis of 1-Cyano-3-arylindolizines: Construction of Pyrroles via DDQ-Mediated Ring Closure of Cyclopropyl Pyridines. Adv. Synth. Catal. 2022, 364, 3016–3022.
- 83 Wang, Y.; Xie, J.; Lu, P.; Wang, Y. Rh(II)-Catalyzed Synthesis of 5H-Isochromeno[3,4-b]Indolizines from 4-Diazoisochroman-3-Imines and Pyridines. Org. Biomol. Chem. 2022, 20, 8484–8488.
- 84 Wang, E.; Luo, J.; Zhang, L.; Zhang, J.; Jiang, Y. Copper-Catalyzed Oxidative [3 + 2] Cycloaddition of Enamines and Pyridotriazoles toward Indolizines. Org. Lett. 2024, 26, 1249–1254.
- 85 Hou, X.; Wang, R.; Fang, F.; Qu, Z.; Zhou, J.; Yu, T.; Wang, D.; Liu, H.; Zhou, Y. Rh(III)-Catalyzed C-H Activation/Annulation for the Construction of Quinolizinones and Indolizines. Org. Lett. 2024, 26, 4451–4456.
- 86 Lu, C.; Ye, M.; Li, M.; Zhang, Z.; He, Y.; Long, L.; Chen, Z. Transition- Metal-Switchable Divergent Synthesis of Nitrile-Containing Pyrazolo [1,5-a] Pyridines and Indolizines. Chin. Chem. Lett. 2021, 32, 3967–3971.
- 87 Roy, S. A.; Zgheib, J.; Zhou, C.; Arndtsen, B. A. Palladium Catalyzed Synthesis of Indolizines via the Carbonylative Coupling of Bromopyridines, Imines and Alkynes. Chem. Sci. 2021, 12, 2251–2256.
- 88 Ahmad, F.; Ranga, P. K.; Pankhade, Y. A.; Fatma, S.; Gouda, A.; Anand, R. V. Pd(II)-Catalyzed Annulation of Terminal Alkynes with 2-Pyridinyl-Substituted P-quinone Methides: Direct Access to Indolizines. Chem. Commun. 2022, 58, 13238–13241.
- 89 Ahmad, F.; Ranga, P. K.; Fatma, S.; Kumar, A.; Anand, R. V. Cu(II)- Catalyzed [3+2]-Annulation of 2-Pyridinyl-substituted p-Quinone Methides with Enaminones: Access to Functionalized Indolizine Derivatives. Adv. Synth. Catal. 2023, 365, 3271–3276.
- 90 Yang, Q.-L.; Ma, R.-C.; Li, Z.-H.; Li, W.-W.; Qu, G.-R.; Guo, H.-M. Electrochemically-Initiated Intramolecular 1,2-Amino Oxygenation of Alkynes: Facile Access to Formyl-and Acyl-Substituted Indolizines. Org. Chem. Front. 2022, 9, 4990–4997.
- 91 Zhao, P.; Yu, Z.-C.; Wang, L.-F.; Zhou, Y.; Wu, Y.-D.; Ma, Y.; Wu, A.-X. I2-Promoted In Situ Cyclization–Rethiolation Reaction: Synthesis of 2-Aliphatic- or Aromatic-Substituted Indolizines. J. Org. Chem. 2022, 87, 15197–15209.
- 92 Zeng, K.; Pandit, N. K.; Oliveira, J. C. A.; Dechert, S.; Ackermann, L.; Zhang, K. Weak-Coordination-Auxiliary Aminocatalysis Enables Directed [3+2] Cyclization for 2-Acylindolizines. Green Chem. 2024, 26, 5253–5259.
- 93 Zhang, C.; Wang, W.; Zhu, X.; Chen, L.; Luo, H.; Guo, M.; Liu, D.; Liu, F.; Zhang, H.; Li, Q.; Lin, J. Synthesis of Indolizines via Tf2O-Mediated Cascade Reaction of Pyridyl-enaminones with Thiophenols/Thioalcohols. Org. Lett. 2023, 25, 1192–1197.
- 94
Day, J.; McKeever-Abbas, B.; Dowden, J. Stereoselective Synthesis of Tetrahydroindolizines through the Catalytic Formation of Pyridinium Ylides from Diazo Compounds. Angew. Chem. Int. Ed. 2016, 128, 5903–5907.
10.1002/ange.201511047 Google Scholar
- 95 Douglas, T.; Pordea, A.; Dowden, J. Iron-Catalyzed Indolizine Synthesis from Pyridines, Diazo Compounds, and Alkynes. Org. Lett. 2017, 19, 6396–6399.
- 96 Chen, R.; Zhao, Y.; Sun, H.; Shao, Y.; Xu, Y.; Ma, M.; Ma, L.; Wan, X. In Situ Generation of Quinolinium Ylides from Diazo Compounds: Copper-Catalyzed Synthesis of Indolizine. J. Org. Chem. 2017, 82, 9291–9304.
- 97 Wang, W.; Han, J.; Sun, J.; Liu, Y. CuBr-Catalyzed Aerobic Decarboxylative Cycloaddition for the Synthesis of Indolizines under Solvent- Free Conditions. J. Org. Chem. 2017, 82, 2835–2842.
- 98 Liu, S.-W.; Gao, Y.-J.; Shi, Y.; Zhou, L.; Tang, X.; Cui, H.-L. Synthesis of Benzoindolizines through 1,5-Electrocyclization/Oxidation Cascades. J. Org. Chem. 2018, 83, 13754–13764.
- 99 Asghari, S.; Qandalee, M.; Behboodi, V.; Gorji, A. N.; Pasha, G. F. Chemoselective Synthesis of Novel Aminoindolizines Using Aminopyridines, Acetylenic Diesters and α-Halo Ketones. Chin. Chem. Lett. 2016, 27, 361–364.
- 100 Jin, T.; Tang, Z.; Hu, J.; Yuan, H.; Chen, Y.; Li, C.; Jia, X.; Li, J. Iron-Catalyzed Aerobic Oxidation and Annulation Reaction of Pyridine and α-Substituted Allenoate toward Functionalized Indolizine. Org. Lett. 2018, 20, 413–416.
- 101 Nechaev, V.; Cherkaev, G. V. Radical and Ionic Reactions of Indolizin- 1-ols: Synthesis of 3-Arylsulfanyl-, 3-(Tropon-2-yl)-and 3-(Tropolon-5- ylazo)-1-hydroxyindolizines from 3, 3-Difluorocyclopropenes. J. Org. Chem. 2021, 86, 7687–7700.
- 102 Nechaev, V.; Cherkaev, G. V.; Solyev, P. N.; Boev, N. V. Synthesis and Aerobic Dehydrogenation of Indolizin-1-ol Derivatives. J. Org. Chem. 2021, 86, 4220–4235.
- 103 Nechaev, V.; Cherkaev, G. V.; Sheremetev, A. B. Unique Pseudo- Cross-Conjugated Mesomeric Betaines via an Iodate-Promoted Reaction of 3,3-Difluorocyclopropenes, Pyridines, and Anilines. J. Org. Chem. 2022, 87, 652–669.
- 104 Chen, H.-R.; Hu, Z.-Y.; Qin, H.-L.; Tang, H. A Novel Three-Component Reaction for Constructing Indolizine-Containing Aliphatic Sulfonyl Fluorides. Org. Chem. Front. 2021, 8, 1185–1189.
- 105 Yavari, I.; Naeimabadi, M.; Halvagar, M. R. FeCl3-Catalyzed Formation of Indolizine Derivatives via the 1,3-Dipolar Cycloaddition Reaction between Azomethine Ylides and Chalcones or Dibenzylideneacetones. Tetrahedron Lett. 2016, 57, 3718–3721.
- 106 Yavari, I.; Ghafouri, K.; Naeimabadi, M.; Halvagar, M. R. A Synthesis of Functionalized 2-Indolizin-3-yl-1,3-benzothiazoles from 1-(1,3-Benzothiazol-2-ylmethyl)pyridinium Iodide and Acetylenic Esters. Synlett 2018, 29, 243–245.
- 107 Yavari, I.; Naeimabadi, M. Synthesis of 3-(quinolin-2-yl) Indolizines through Iodine-Mediated sp3 C-H Functionalization of Azaarenes. Synth. Commun. 2018, 48, 632–637.
- 108 Fang, Y.; He, L.; Pan, W.; Yang, Y. Iodine-Mediated One-Pot Synthesis of C-3 Acylated Indolizines from Pyridines, Aryl Methyl Ketones and Acrylate Derivatives. Tetrahedron 2019, 75, 3767–3771.
- 109 Zhang, Q.; Wang, B.; Ma, H.; Ablajan, K. Transition-Metal-Free Catalyzed [3+2] Cycloadditions/Oxidative Aromatization Reactions for The Synthesis of Annulated Indolizines. New J. Chem. 2019, 43, 17000–17003.
- 110 Zhang, X.-J.; Wang, Z.; Zhang, H.; Gao, J.-J.; Yang, K. R.; Fan, W.-Y.; Wu, R. X.; Feng, M.-L.; Zhu, W.; Zhu, Y.-P. Iodine-Mediated Domino Cyclization for One-Pot Synthesis of Indolizine-Fused Chromones via Metal-Free sp3 C-H Functionalization. J. Org. Chem. 2022, 87, 835–845.
- 111 Malviya, B. K.; Jassal, A. K.; Karnatak, M.; Verma, V. P.; Sharma, S. Electro-Oxidative sp3 C–H Bond Functionalization and Annulation Cascade: Synthesis of Novel Heterocyclic Substituted Indolizines. J. Org. Chem. 2022, 87, 2898–2911.
- 112 Li, X.; Chen, Z.; Chen, W.; Xie, X.; Zhou, H.; Liao, Y.; Yu, F.; Huang, J. B2pin2-Mediated Cascade Cyclization/Aromatization Reaction: Facial Access to Functionalized Indolizines. Org. Lett. 2022, 24, 7372–7377.
- 113 Hu, D. X.; Withall, D. M.; Challis, G. L.; Thomson, R. J. Structure, chemical synthesis, and biosynthesis of prodiginine natural products. Chem. Rev. 2016, 116, 7818–7853.
- 114 Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932.
- 115 Orłowski, R.; Gryko, D.; Gryko, D. T. Synthesis of corroles and their heteroanalogs. Chem. Rev. 2017, 117, 3102–3137.
- 116 Eftekhari-Sis, B.; Zirak, M.; Akbari, A. Electro-Oxidative sp3 C-H Bond Functionalization and Annulation Cascade: Synthesis of Novel Heterocyclic Substituted Indolizines. Chem. Rev. 2013, 113, 2958–3043.
- 117 Ghosh, A.; Shee, S.; Barik, S.; Gonnade, R. G.; Biju, A. T. Enantioselective Synthesis of 5,6-Dihydroindolizines by N-Heterocyclic Carbene (NHC)-Catalyzed Core-Structure-Inspired Strategy of Azolium-Enolate Cascade. Org. Lett. 2021, 23, 5223–5228.
- 118 Huang, W.; Chen, S.; Yang, J.; EL-Harairy, A.; Wang, X.; Li, M.; Gu, Y. Modular Synthesis of Bicyclic and Tricyclic (Aza-) Arenes from Nucleophilic (Aza-)Arenes with Electrophilic Side Arms via [4+2] Annulation Reactions. Adv. Synth. Catal. 2019, 361, 4369–4378.
- 119 Li, X.; Xie, X.; Liu, Y. Gold(I)-Catalyzed Cascade Hydroarylation/Cycloaromatization to Indolizines via Pyridine Ring Construction. J. Org. Chem. 2016, 81, 3688–3699.
- 120 Li, X.; Zhao, J.; Xie, X.; Liu, Y. Synthesis of Functionalized Indolizines via Gold(I)-Catalyzed Intramolecular Hydroarylation/aromatization of Pyrrole-Ynes. Org. Biomol. Chem. 2017, 15, 8119–8133.
- 121 Liu, R.; Wang, Q.; Wei, Y.; Shi, M. Synthesis of Indolizine Derivatives Containing Eight-membered Ring by a Gold-catalyzed Two-fold Hydroarylation of Diynes. Chem. Commun. 2018, 54, 1225–1228.
- 122 Zhou, B.; Guo, M.; Pan, Q.; Zhou, M.; Xu, L.; Rao, Y.; Wang, K.; Yin, B.; Zhou, J.; Song, J. Rhodium-Catalyzed Annulation of Pyrrole Substituted BODIPYs with Alkynes to Access π-Extended Polycyclic Heteroaromatic Molecules and NIR Absorption. Org. Chem. Front. 2021, 8, 868–875.
- 123 Joshi, D. R.; Kim, I. Michael-Aldol Double Elimination Cascade to Make Pyridines: Use of Chromone for the Synthesis of Indolizines. J. Org. Chem. 2021, 86, 10235–10248.
- 124 Zhong, W.; Zhu, H.; Zou, H. One-Pot Cascade Approach to 5,6-Dihydroindolizines and Indolizines from Pyrrole-2-Carbaldehydes and Nitroethylenes. Tetrahedron 2017, 73, 3181–3187.
- 125 Shao, N.; Li, J.; Zhu, H.; Zhang, S.; Zou, H. Functionalized N-containing Heterocyclic Scaffolds Derived from N-Substituted Pyrroles via Inter-and Intramolecular Annulations. Tetrahedron 2018, 74, 6088–6094.
- 126 Li, J.; Zhang, S.; Zou, H. One-Pot Chemoselective Domino Condensation to form a Fused Pyrrolo-Pyrazino-Indolizine Framework: Discovery of Novel AIE Molecules. Org. Chem. Front. 2020, 7, 1218–1223.
- 127 Kim, S.; Lee, J. H.; Yoon, S. H.; Kim, I. A regioselective [4+2] Annulation Approach to 5-Acylindolizine-7-Carbonitriles: Generation of Poly-substituted Pyridines. Org. Biomol. Chem. 2021, 19, 5806–5817.
- 128 Gong, M.; Guo, J.; Jiang, P.; Zhang, Y.; Fu, Z.; Huang, W. Facile Synthesis of Polysubstituted Indolizines via One-Pot Reaction of 1-Acetylaryl 2-Formylpyrroles and Enals. Chem.-Asian J. 2020, 15, 352–355.
- 129 Zhang, Y.-Y.; Li, L.; Ma, A.-J.; Wang, W.-F.; Peng, J.-B. Base-Promoted [4+2] Annulation of Pyrrole-2-Carbaldehyde Derivatives with β,γ-Unsaturated α-ketoesters: Syntheses of 5,6-Dihydroindolizines. Org. Biomol. Chem. 2022, 20, 8633–8637.
- 130 Gallardo-Alfonzo, S.; Cortés-Garcia, C. J.; Mejía-Farfán, I.; López, Y.; Mojica, M.; Contreras-Celedón, C.; Chacón-García, L. A Two-Step Synthesis of a Novel 7,8-Dihydro-5,8-Ethanoindolizine-6,9 (5H)-Dione. Synlett 2021, 32, 185–191.
- 131 Joshi, D. R.; Seo, Y.; Heo, Y.; Park, S. H.; Lee, Y.; Namkung, W.; Kim, I. Domino [4+2] Annulation Access to Quinone-indolizine Hybrids: Anticancer N-fused polycycles. J. Org. Chem. 2020, 85, 10994–11005.
- 132 Palani, V.; Perea, M. A.; Gardner, K. E.; Sarpong, R. A Pyrone Remodeling Strategy to Access Diverse Heterocycles: Application to the Synthesis of Fascaplysin Natural Products. Chem. Sci. 2021, 12, 1528–1534.
- 133 Duan, G.; Liu, H.; Zhang, L.; Yuan, C.; Li, Y.; Ge, Y. Access to 6-Hydroxy Indolizines and Related Imidazo [1, 5-a] Pyridines Through the SN 2 Substitution/Condensation/Tautomerization Cascade Process. RSC Adv. 2021, 11, 25624–25627.
- 134 Joshi, D. R.; Kim, I. Synthesis of Poly-Functionalized Indolizines via [5+1] Annulative Access to Pyridines. Adv. Synth. Catal. 2021, 363, 5330–5335.
- 135 Escalante, C. H.; Carmona-Hernández, F. A.; Hernández-López, A.; Martínez-Mora, E. I.; Delgado, F.; Tamariz, J. Cascade Synthesis of Indolizines and Pyrrolo [1,2-a] Pyrazines from 2-Formyl-1-Propargylpyrroles. Org. Biomol. Chem. 2022, 20, 396–409.
- 136 Lepitre, T.; Le Biannic, R.; Othman, M.; Lawson, A. M.; Daïch, A. Metal-free Cascade Approach toward Polysubstituted Indolizines from Chromone-based Michael Acceptors. Org. Lett. 2017, 19, 1978–1981.
- 137 Raj, S. K.; Tan, C. K.; Chen, L. Y.; Cheng, M. J.; Liu, R. S. Gold-Catalyzed Bicyclic Annulations of 4-Methoxy-1,2-Dienyl-5-ynes with Isoxazoles to form Indolizine Derivatives via an Au-π-allene Intermediate. Chem. Sci. 2019, 10, 6437–6442.
- 138 Sirindil, F.; Golling, S.; Lamare, R.; Weibel, J. M.; Pale, P.; Blanc, A. Synthesis of Indolizine and Pyrrolo[1,2-a]azepine Derivatives via a Gold(I)-Catalyzed Three-Step Cascade. Org. Lett. 2019, 21, 8997–9000.
- 139 Tang, R. S.; Chen, L. Y.; Lai, C. H.; Chuang, T. H. Palladium-Catalyzed Stereoselective Aza-Wacker-Heck Cyclization: One-Pot Stepwise Strategy toward Tetracyclic Fused Heterocycles. Org. Lett. 2020, 22, 9337–9341.
- 140 Xu, X.; Feng, H.; Zhang, X.; Song, L.; Van Meervelt, L.; Van der Eycken, J.; Harvey, J. N.; Van der Eycken, E. H. Pd-catalyzed Ring Restructuring of Oxazolidines with Alkenes Leading to Fused Polycyclic Indolizines. Org. Lett. 2022, 24, 1232–1236.
- 141 Karjee, P.; Debnath, B.; Mandal, S.; Saha, S.; Punniyamurthy, T. One-pot C-N/C-C Bond Formation and Oxidation of Donor-acceptor Cyclopropanes with Tetrahydroisoquinolines: Access to Benzo-fused Indolizines. Chem. Commun. 2024, 60, 4068–4071.




