Dual Photoredox and Titanium Catalyzed Regioselective Allenylation of Aldehydes via Reductive Radical-Polar Crossover
Zulin Xiao
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
These authors contributed equally.
Search for more papers by this authorWenzhe Shang
Dalian University of Technology, School of Chemistry, Dalian, Liaoning, 116024 China
These authors contributed equally.
Search for more papers by this authorTao Huang
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
These authors contributed equally.
Search for more papers by this authorLei He
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
Search for more papers by this authorJingyao Li
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
Search for more papers by this authorShen Luo
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
Search for more papers by this authorXiaoxia He
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
Search for more papers by this authorXiang Li
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
Search for more papers by this authorCorresponding Author
Fusheng Li
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
E-mail: [email protected]Search for more papers by this authorZulin Xiao
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
These authors contributed equally.
Search for more papers by this authorWenzhe Shang
Dalian University of Technology, School of Chemistry, Dalian, Liaoning, 116024 China
These authors contributed equally.
Search for more papers by this authorTao Huang
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
These authors contributed equally.
Search for more papers by this authorLei He
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
Search for more papers by this authorJingyao Li
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
Search for more papers by this authorShen Luo
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
Search for more papers by this authorXiaoxia He
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
Search for more papers by this authorXiang Li
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
Search for more papers by this authorCorresponding Author
Fusheng Li
School of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, 421001 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
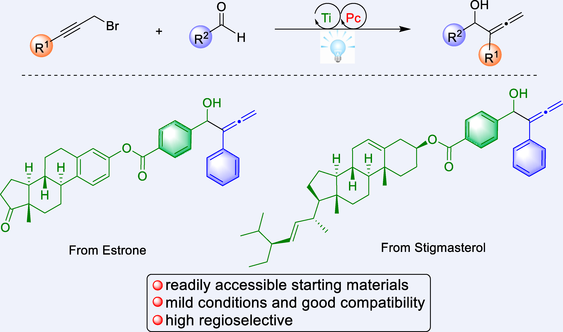
The direct reductive coupling of carbonyl compounds with propargyl halides is a powerful and reliable tool in the synthesis of α-allenols. However, stoichiometric metal reductants, harsh reaction conditions and a variety of additional additives are required in the traditional strategies. Additionally, the reactivity and regioselectivity control remains an elusive challenge. Herein, we developed the Ti-catalyzed regioselective reductive coupling of readily available aldehydes and racemic propargyl bromides to rapidly access a wide range of α-allenols. This method proceed efficiently in a reductive radical-polar crossover manner featuring mild conditions, excellent regioselectivity control, broad substrate scope, and eco-friendliness. Preliminary mechanistic studies support the radical-involved catalytic cycle. And the DFT calculations demonstrate that the regioselectivity is determined by the Zimmerman-Traxler-type transition states.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400705-sup-0001-supinfo.pdfPDF document, 18.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Huang, X.; Ma, S. Allenation of Terminal Alkynes with Aldehydes and Ketones. Acc. Chem. Res. 2019, 52, 1301–1312.
- 2 Mascareñas, J. L.; Varela, I.; López, F. Allenes and Derivatives in Gold(I)- and Platinum(II)-Catalyzed Formal Cycloadditions. Acc. Chem. Res. 2019, 52, 465–479.
- 3 Alonso, J. M.; Almendros, P. Deciphering the Chameleonic Chemistry of Allenols: Breaking the Taboo of a Onetime Esoteric Functionality. Chem. Rev. 2021, 121, 4193–4252.
- 4 Zhang, F.; Guo, X.; Zeng, X.; Wang, Z. Catalytic Enantioconvergent Allenylation of Aldehydes with Propargyl Halides. Angew. Chem. Int. Ed. 2022, 61, e202117114.
- 5 Yu, L.; Wang, H.; Akhmedov, N. G.; Sowa, C.; Liu, K.; Kim, H.; Williams, L. Direct Entry to 4,10-Didesmethyl (9S)-Dihydroerythronolide A via Catalytic Allene Osmylation. Org. Lett. 2016, 18, 2868–2871.
- 6 Deliaval, M.; Jayarajan, R.; Eriksson, L.; Szabó, K. J. Three-Component Approach to Densely Functionalized Trifluoromethyl Allenols by Asymmetric Organocatalysis. J. Am. Chem. Soc. 2023, 145, 10001–10006.
- 7 Huang, X.; Cao, T.; Han, Y.; Jiang, X.; Lin, W.; Zhang, J.; Ma, S. General CuBr2-Catalyzed Highly Enantioselective Approach for Optically Active Allenols From Terminal Alkynols. Chem. Commun. 2015, 51, 6956–6959.
- 8 Wang, G.; Liu, X.; Chen, Y.; Yang, J.; Li, J.; Lin, L.; Feng, X. Diastereoselective and Enantioselective Alleno-Aldol Reaction of Allenoates with Isatins to Synthesis of Carbinol Allenoates Catalyzed by Gold. Acs Catal. 2016, 6, 2482–2486.
- 9 Tap, A.; Blond, A.; Wakchaure, V. N.; List, B. Chiral Allenes via Alkynylogous Mukaiyama Aldol Reaction. Angew. Chem. Int. Ed. 2016, 55, 8962–8965.
- 10 Tang, Y.; Xu, J.; Yang, J.; Lin, L.; Feng, X.; Liu, X. Asymmetric Three- Component Reaction for the Synthesis of Tetrasubstituted Allenoates via Allenoate-Copper Intermediates. Chem 2018, 4, 1658–1672.
- 11 Zhong, F.; Xue, Q.; Yin, L. Construction of Chiral 2,3-Allenols through a Copper(I)-Catalyzed Asymmetric Direct Alkynylogous Aldol Reaction. Angew. Chem. Int. Ed. 2020, 59, 1562–1566.
- 12 Law, C.; Kativhu, E.; Wang, J.; Morken, J. P. Diastereo- and Enantioselective 1,4-Difunctionalization of Borylenynes by Catalytic Conjunctive Cross-Coupling. Angew. Chem. Int. Ed. 2020, 59, 10311–10315.
- 13 Xu, G.; Wang, Z.; Shao, Y.; Sun, J. Copper-Catalyzed Tandem Cross-Coupling and Alkynylogous Aldol Reaction: Access to Chiral Exocyclic Α-Allenols. Org. Lett. 2021, 23, 5175–5179.
- 14 Mukaiyama, T.; Harada, T. A Convenient Synthesis of Α-Hydroxyallenes by the Reaction of Propargyl Iodides with Aldehydes in the Presence of Stannous Halide. Chem. Lett. 1981, 10, 621–624.
- 15 Ma, S.; Gao, W. Efficient Synthesis of 4-(2’-Alkenyl)-2,5-Dihydrofurans and 5, 6-Dihydro-2H-Pyrans via the Pd-Catalyzed Cyclizative Coupling Reaction of 2,3- Or 3,4-Allenols with Allylic Halides. J. Org. Chem. 2002, 67, 6104–6112.
- 16 Kong, W.; Fu, C.; Ma, S. Indium and Zinc-Mediated Barbier-Type Addition Reaction of 2,3-Allenals with Allyl Bromide: An Efficient Synthesis of 1,5,6-Alkatrien-4-Ols. Org. Biomol. Chem. 2008, 6, 4587–4592.
- 17 Zhang, F.; Guo, X.; Zeng, X.; Wang, Z. Asymmetric 1,4-Functionalization of 1,3-Enynes via Dual Photoredox and Chromium Catalysis. Nat. Commun. 2022, 13, 5036.
- 18 Guo, X.; Shi, Z.; Zhang, F.; Wang, Z. Cr-Catalyzed Regio-, Diastereo-, and Enantioselective Reductive Couplings of Ketones and Propargyl Halides. ACS Catal. 2023, 13, 3170–3178.
- 19 Pitzer, L.; Schwarz, J. L.; Glorius, F. Reductive Radical-Polar Crossover: Traditional Electrophiles in Modern Radical Reactions. Chem. Sci. 2019, 10, 8285–8291.
- 20 Sharma, S.; Singh, J.; Sharma, A. Visible Light Assisted Radical-Polar/ Polar-Radical Crossover Reactions in Organic Synthesis. Adv. Synth. Catal. 2021, 363, 3146–3169.
- 21 Jiang, X.; Jiang, H.; Yang, Q.; Cheng, Y.; Lu, L.; Tunge, J. A.; Xiao, W. Photoassisted Cobalt-Catalyzed Asymmetric Reductive Grignard-Type Addition of Aryl Iodides. J. Am. Chem. Soc. 2022, 144, 8347–8354.
- 22 Jiang, H.; He, X.; Jiang, X.; Zhao, W.; Lu, L.; Cheng, Y.; Xiao, W. Photoinduced Cobalt-Catalyzed Desymmetrization of Dialdehydes to Access Axial Chirality. J. Am. Chem. Soc. 2023, 145, 6944–6952.
- 23 Liang, T.; Wu, Y.; Sun, J.; Li, M.; Zhao, H.; Zhang, J.; Zheng, G.; Zhang, Q. Visible Light-Mediated Cobalt and Photoredox Dual-Catalyzed Asymmetric Reductive Coupling for Axially Chiral Secondary Alcohols. Chin. J. Chem. 2023, 41, 3253–3260.
- 24 Wu, J.; Xu, X.; Duan, C.; Chen, S.; Wang, D.; Fang, L.; Tang, W.; Li, F. Diastereoselective 1,2-Difunctionalization of 1,3-Enynes Enabled by Merging Photoexcited Hantzsch Ester with Chromium Catalysis. Org. Chem. Front. 2024, 11, 284–289.
- 25 Wang, F.; Wang, D.; Zhou, Y.; Liang, L.; Lu, R.; Chen, P.; Lin, Z.; Liu, G. Divergent Synthesis of CF3-Substituted Allenyl Nitriles by Ligand-Controlled Radical 1,2-and 1,4-Addition to 1,3-Enynes. Angew. Chem. Int. Ed. 2018, 57, 7140–7145.
- 26 Song, Y.; Fu, C.; Ma, S. Copper-Catalyzed Syntheses of Multiple Functionalizatized Allenes via Three-Component Reaction of Enynes. ACS Catal. 2021, 11, 10007–10013.
- 27 Zhang, K. F.; Bian, K. J.; Li, C.; Sheng, J.; Li, Y.; Wang, X. S. Nickel-Catalyzed Carbofluoroalkylation of 1, 3-Enynes to Access Structurally Diverse Fluoroalkylated Allenes. Angew. Chem. Int. Ed. 2019, 58, 5069–5074.
- 28 Zhu, X.; Deng, W.; Chiou, M.; Ye, C.; Jian, W.; Zeng, Y.; Jiao, Y.; Ge, L.; Li, Y.; Zhang, X.; Bao, H. Copper-Catalyzed Radical 1,4-Difunctionalization of 1,3-Enynes with Alkyl Diacyl Peroxides and N-Fluorobenzenesulfonimide. J. Am. Chem. Soc. 2018, 141, 548–559.
- 29 Zeng, Y.; Chiou, M.; Zhu, X.; Cao, J.; Lv, D.; Jian, W.; Li, Y.; Zhang, X.; Bao, H. Copper-Catalyzed Enantioselective Radical 1,4-Difunctionalization of 1, 3-Enynes. J. Am. Chem. Soc. 2020, 142, 18014–18021.
- 30
Dong, X. Y.; Zhan, T. Y.; Jiang, S. P.; Liu, X. D.; Ye, L.; Li, Z. L.; Gu, Q. S.; Liu, X. Y. Copper-Catalyzed Asymmetric Coupling of Allenyl Radicals with Terminal Alkynes to Access Tetrasubstituted Allenes. Angew. Chem. Int. Ed. 2021, 133, 2188–2192.
10.1002/ange.202013022 Google Scholar
- 31 Liu, W.; Liu, C.; Wang, M.; Kong, W. Modular Synthesis of Multifunctionalized CF3-Allenes through Selective Activation of Saturated Hydrocarbons. ACS Catal. 2022, 12, 10207–10221.
- 32 Hu, D.; Gao, Q.; Dai, J.; Cui, R.; Li, Y.; Li, Y.; Zhou, X.; Bian, K.; Wu, B.; Zhang, K. Visible-Light-Induced, Autopromoted Nickel-Catalyzed Three-Component Arylsulfonation of 1, 3-Enynes and Mechanistic Insights. Sci. China Chem. 2022, 65, 753–761.
- 33 Inoue, M.; Nakada, M. Studies Into Asymmetric Catalysis of the Nozaki–Hiyama Allenylation. Angew. Chem. Int. Ed. 2006, 45, 252–255.
- 34 Xia, G.; Yamamoto, H. Catalytic Enantioselective Allenylation Reactions of Aldehydes with Tethered Bis(8-Quinolinolato) (Tbox) Chromium Complex. J. Am. Chem. Soc. 2007, 129, 496–497.
- 35 Coeffard, V.; Aylward, M.; Guiry, P. J. First Regio- And Enantioselective Chromium-Catalyzed Homoallenylation of Aldehydes. Angew. Chem. Int. Ed. 2009, 48, 9152–9155.
- 36 Padial, N. M.; Hernández-Cervantes, C.; Muñoz-Bascón, J.; Roldán-Molina, E.; García-Martínez, M.; Ruiz-Muelle, A. B.; Rosales, A.; Álvarez-Corral, M.; Muñoz-Dorado, M.; Rodríguez-García, I.; Oltra, J. E. Ti-Catalyzed Synthesis of Exocyclic Allenes On Oxygen Heterocycles. Eur. J. Org. Chem. 2017, 2017, 639–645.
- 37 Lang, X.; Zhao, J.; Chen, X. Cooperative Photoredox Catalysis. Chem. Soc. Rev. 2016, 45, 3026–3038.
- 38 Chan, A. Y.; Perry, I. B.; Bissonnette, N. B.; Buksh, B. F.; Edwards, G. A.; Frye, L. I.; Garry, O. L.; Lavagnino, M. N.; Li, B. X.; Liang, Y.; Mao, E.; Millet, A.; Oakley, J. V.; Reed, N. L.; Sakai, H. A.; Seath, C. P.; MacMillan, D. W. C. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev. 2022, 122, 1485–1542.
- 39 Zhang, Z.; Slak, D.; Krebs, T.; Leuschner, M.; Schmickler, N.; Kuchuk, E.; Schmidt, J.; Domenianni, L. I.; Kleine Büning, J. B.; Grimme, S.; Vöhringer, P.; Gansäuer, A. A Chiral Titanocene Complex as Regiodivergent Photoredox Catalyst: Synthetic Scope and Mechanism of Catalyst Generation. J. Am. Chem. Soc. 2023, 145, 26667–26677.
- 40 Schmidt, J.; Domenianni, L. I.; Leuschner, M.; Gansäuer, A.; Vöhringer, P. Observing the Entry Events of a Titanium-Based Photoredox Catalytic Cycle in Real Time. Angew. Chem. Int. Ed. 2023, 62, e202307178.
- 41 Li, F.; Chen, Y.; Lin, S.; Shi, C.; Li, X.; Sun, Y.; Guo, Z.; Shi, L. Visible-Light-Mediated Barbier Allylation of Aldehydes and Ketones via Dual Titanium and Photoredox Catalysis. Org. Chem. Front. 2020, 7, 3434–3438.
- 42 Gualandi, A.; Calogero, F.; Mazzarini, M.; Guazzi, S.; Fermi, A.; Bergamini, G.; Cozzi, P. G. Cp2TiCl2-Catalyzed Photoredox Allylation of Aldehydes with Visible Light. ACS Catal. 2020, 10, 3857–3863.
- 43 Li, F.; Lin, S.; Chen, Y.; Shi, C.; Yan, H.; Li, C.; Wu, C.; Lin, L.; Duan, C.; Shi, L. Photocatalytic Generation of Π-Allyltitanium Complexes via Radical Intermediates. Angew. Chem. Int. Ed. 2021, 60, 1561–1566.
- 44 Gansäuer, A.; Kube, C.; Daasbjerg, K.; Sure, R.; Grimme, S.; Fianu, G. D.; Sadasivam, D. V.; Flowers, R. A. I. Substituent Effects and Supramolecular Interactions of Titanocene(III) Chloride: Implications for Catalysis in Single Electron Steps. J. Am. Chem. Soc. 2014, 136, 1663–1671.
- 45 Lin, S.; Chen, Y.; Li, F.; Shi, C.; Shi, L. Visible-Light-Driven Spirocyclization of Epoxides Via Dual Titanocene and Photoredox Catalysis. Chem. Sci. 2020, 11, 839–844.
- 46 Calogero, F.; Gualandi, A.; Matteo, M. D.; Potenti, S.; Fermi, A.; Bergamini, G.; Cozzi, P. G. Photoredox Propargylation of Aldehydes Catalytic in Titanium. J. Org. Chem. 2021, 86, 7002–7009.
- 47 Zeng, X.; Zhang, F.; Wang, Z. Cr-Catalyzed Chiral Allenone Synthesis via Sequential Radical–Polar Crossover and Oppenauer Oxidation. Org. Chem. Front. 2023, 10, 310–316.
- 48 Kim, S.; Lee, Y.; Cho, E. J. Photoredox Selective Homocoupling of Propargyl Bromides. J. Org. Chem. 2023, 88, 6382–6389.
- 49 Wang, P.; Chen, J.; Xiao, W. Hantzsch Esters: An Emerging Versatile Class of Reagents in Photoredox Catalyzed Organic Synthesis. Org. Biomol. Chem. 2019, 17, 6936–6951.
- 50 Nikitin, O. M.; Magdesieva, T. V. Electrochemically Induced Titanocene-Mediated Reductive Opening of Epoxides. Mendeleev Commun. 2011, 21, 194–195.
- 51 Liedtke, T.; Spannring, P.; Riccardi, L.; Gansäuer, A. Mechanism- Based Condition Screening for Sustainable Catalysis in Single-Electron Steps by Cyclic Voltammetry. Angew. Chem. Int. Ed. 2018, 57, 5006–5010.




