Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture
- Page: 1645
- First Published: 15 June 2022

This cover picture depicts diversified halogenated secondary metabolites derived from marine microorganisms, while the chlorinated and brominated ones are the two predominant categories. These halogenated compounds with promising bioactivities and good drugability would shed light on the research and development of new candidate drugs. More details are given in the article by Luo et al. on page 1729–1750.
Inside Cover Picture
Inside Cover Picture
- Page: 1646
- First Published: 15 June 2022
This cover picture discribes an efficient three-component [3+2+1] cycloaddition of simple alkenes with two C—H substrates via oxidative dicarbofunctionalization/cyclization sequence. The reaction involves the formation of two C—C bonds and one C—O bond through the cleavage of three C—H bonds in a single operation and a variety of functionalized chromenes were obtained in good yields. More details are given in the article by Liu et al. on page 1681–1686.
Contents
Breaking Report
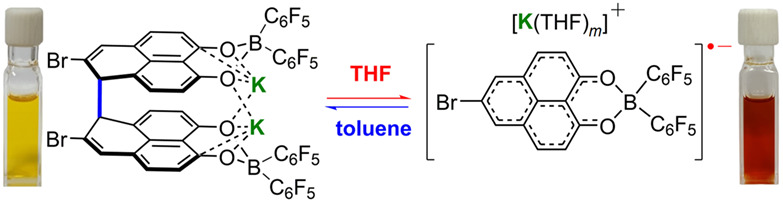
Room-Temperature Reversible σ-Dimerization of a Phenalenyl Radical
- Pages: 1655-1661
- First Published: 16 April 2022
Concise Reports
Protecting-Group-Free One-Step Palladium-Catalyzed Coupling on C25 of Cucurbitacin B Expands Chemical Diversity with Improved Cytotoxicity against A549 Cells
- Pages: 1662-1666
- First Published: 16 April 2022
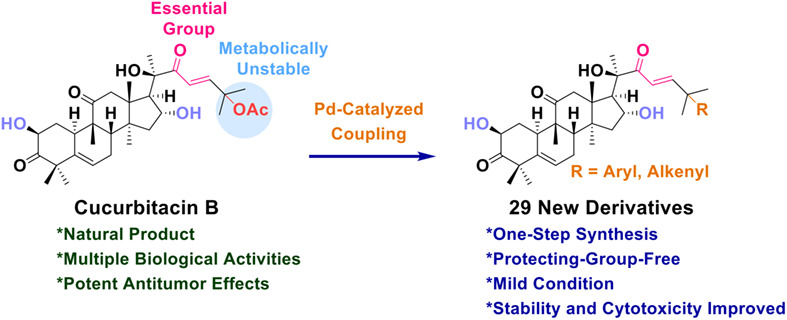
A protecting-group-free palladium-catalyzed allylic coupling of natural product cucurbitacin B with boronic acids was developed, providing a one-step approach to expand the chemical diversity of the C25 position of cucurbitacin B. A library of 29 derivatives was prepared, some of the compounds showed higher cytotoxicity against A549 cells than cucurbitacin B.
Copper-Catalyzed 1,2,5-Trifunctionalization of Terminal Alkynes Using SR as a Transient Directing Group for Radical Translocation
- Pages: 1667-1673
- First Published: 22 April 2022
Kinetic Resolution of 1,2-Diamines via Organocatalyzed Asymmetric Electrophilic Aminations of Anilines
- Pages: 1674-1680
- First Published: 16 April 2022
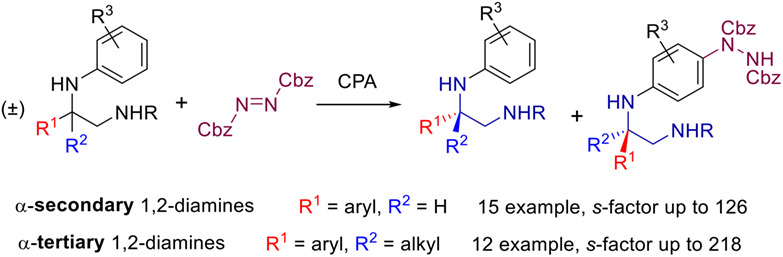
A novel kinetic resolution (KR) protocol for 1,2-diamines has been developed through asymmetric electrophilic aminations of anilines enabled by chiral phosphoric acid catalysis. Notably, 1,2-diamines bearing both α-secondary and α-tertiary amine moieties are compatible with this method, which gave good to high KR performances (with s-factor up to 218).
Copper-Catalyzed [3 + 2 + 1] Cycloaddition of Alkenes with Benzoquinones and Dicarbonyl Compounds via Tandem Oxidative Dicarbofunctionalization/Cyclization Sequence
- Pages: 1681-1686
- First Published: 19 April 2022
![Copper-Catalyzed [3 + 2 + 1] Cycloaddition of Alkenes with Benzoquinones and Dicarbonyl Compounds via Tandem Oxidative Dicarbofunctionalization/Cyclization Sequence](/cms/asset/83e073d2-cdf0-4e15-84ea-ced75cf28a16/cjoc202200113-toc-0001-m.jpg)
An efficient three-component [3 + 2 + 1] cycloaddition of simple alkenes with benzoquinones and dicarbonyl compounds via oxidative dicarbofunctionalization/cyclization sequence has been reported. The copper-catalyzed intermolecular dicarbofunctionalization/cyclization of alkenes proceeds smoothly with two C—H reagents and a variety of functionalized chromenes are obtained involving the formation of two C—C bonds and one C—O bond through the cleavage of three C—H bonds in a single operation.
Electrochemical Synthesis of Sulfonyl Fluorides with Triethylamine Hydrofluoride
- Pages: 1687-1692
- First Published: 16 April 2022
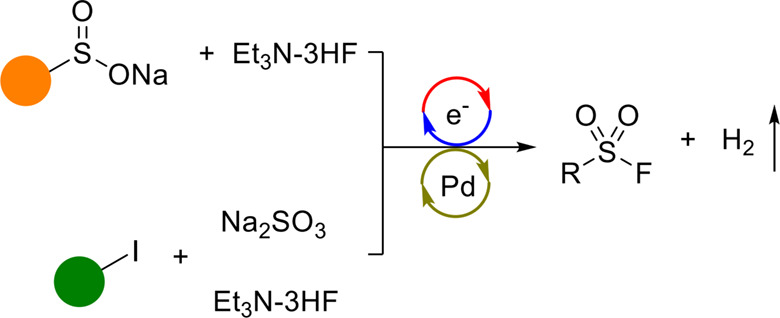
Herein, we report the first method for electrochemical synthesis of sulfonyl fluorides with triethylamine hydrofluoride as a fluorine source. These electricity-driven reactions of odorless sulfinic salts were carried out under oxidant-free conditions. Aryl, aliphatic, and alkenyl sulfonyl fluorides could be accessed by means of this method. In addition, we report a one-pot protocol for Pd-catalyzed C—S cross-coupling and oxidative fluorination under electrochemical conditions. A reaction pathway involving the supporting electrolyte is proposed.
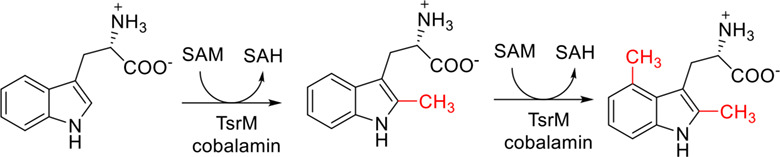
Consecutive Methylation Catalyzed by TsrM, an Atypical Class B Radical SAM Methylase
- Pages: 1693-1698
- First Published: 21 April 2022
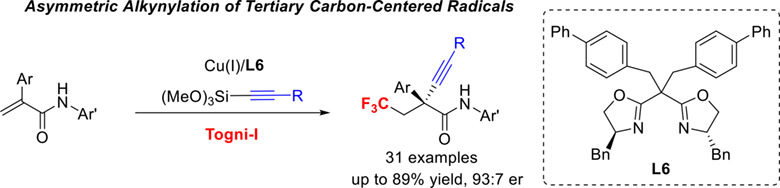
Asymmetric Alkynylation of Tertiary Carbon-Centered Radical via Copper-Catalyzed Radical Relay
- Pages: 1699-1704
- First Published: 20 April 2022
Chemistry Authors Up Close
Cornerstones in Chemistry
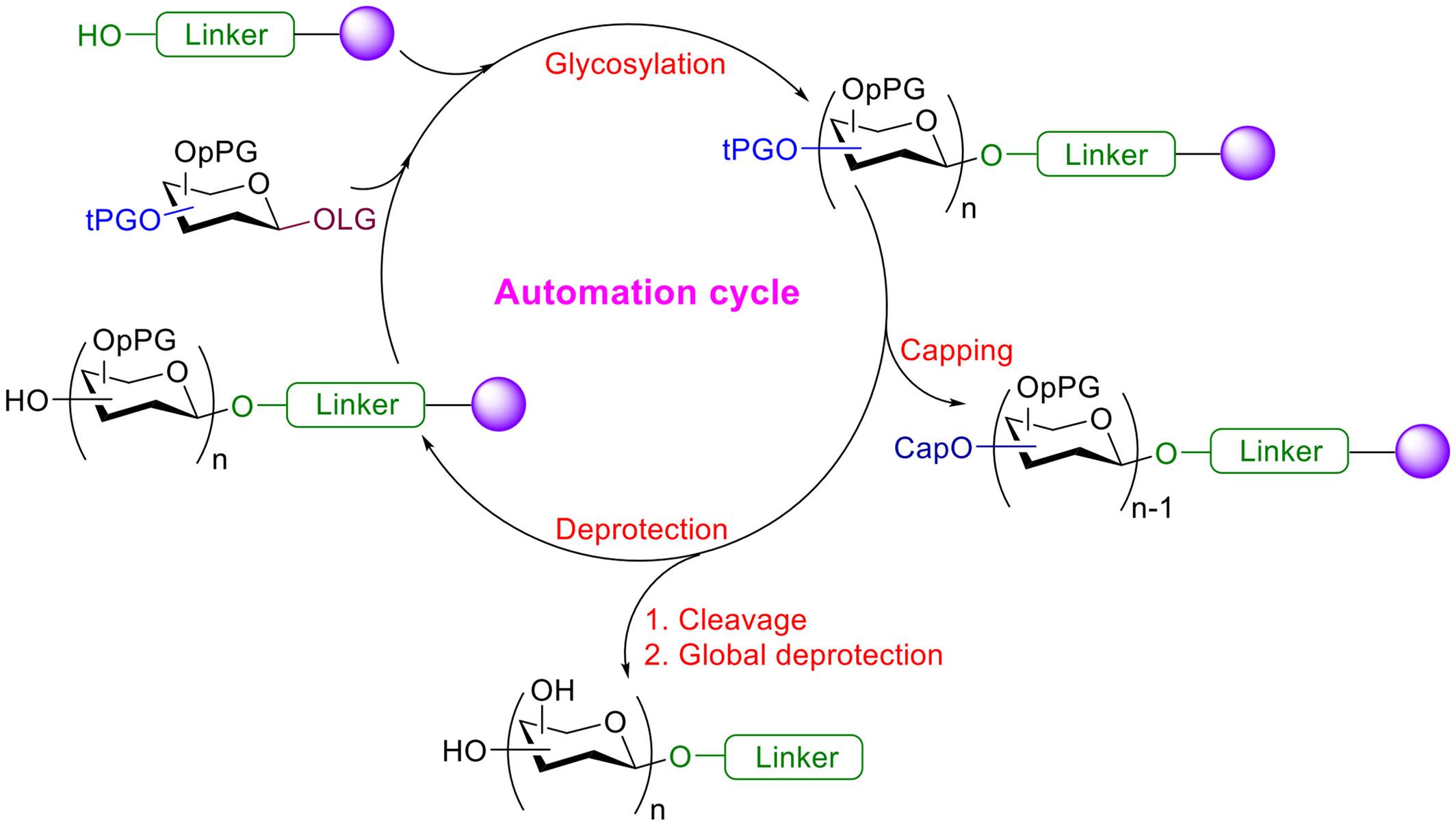
Automated Chemical Solid-Phase Synthesis of Glycans
- Pages: 1714-1728
- First Published: 25 April 2022
Recent Advances
Chemistry, Biosynthesis, and Biological Activity of Halogenated Compounds Produced by Marine Microorganisms
- Pages: 1729-1750
- First Published: 29 March 2022
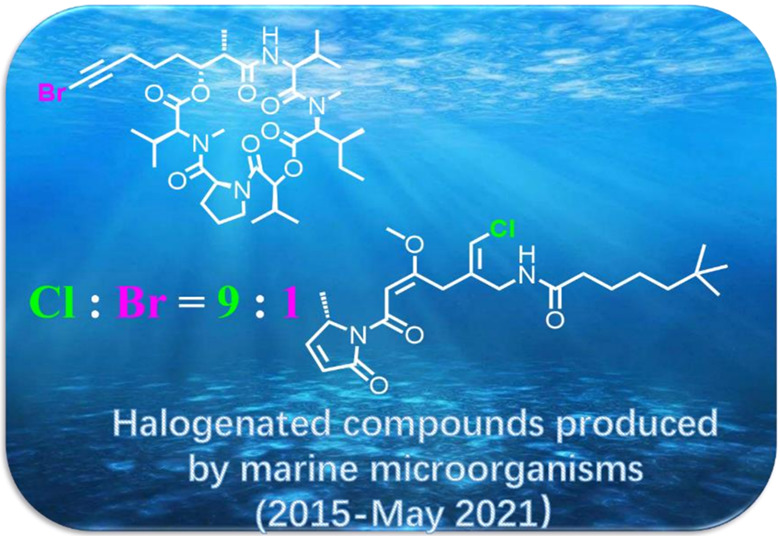
This review aims to summarize the chemistry, biosynthesis, and biological activity of 316 halogenated compounds isolated from marine microorganisms (2015—May 2021). These halogenated compounds originated from 36 genera of fungi (62%) and 9 bacterial strains (38%) were found with diverse structures (polyketides, alkaloids, phenols, and others) and significant bioactivities (cytotoxic, antibacterial, and enzyme inhibitory).
Emerging Topic
Radical-Mediated Selective Functionalization of Unactivated Primary C—H Bonds
- Pages: 1751-1753
- First Published: 02 May 2022
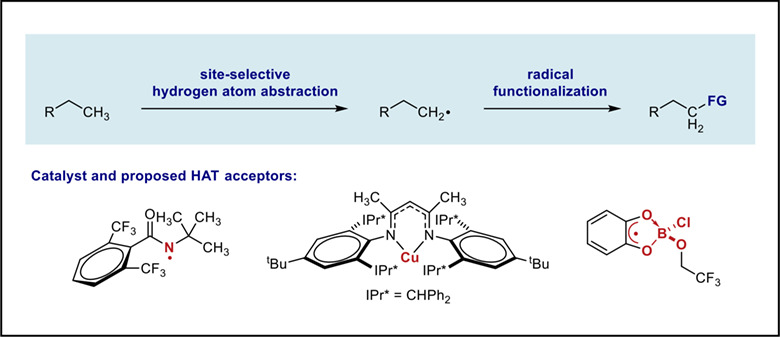
The development of innovative methods capable of overriding the “inherent” site-selectivity in radical-mediated sp3 C—H functionalization is exceedingly attractive and will have important impacts on synthesis. However, the selective activation of unactivated primary C—H bonds remains a daunting challenge. In this Emerging Topic, we highlight the recent advances towards this goal by tuning the steric properties of hydrogen atom acceptors.
Inside Back Cover
Inside Back Cover
- Page: 1755
- First Published: 15 June 2022

The Inside back cover picture shows an unprecedented copper-catalyzed 1,2,5-trifunctionalization of terminal alkynes, which is realized through synergetic hydrogen atom transfer and traceless directing strategy with SR as the transient group. This work features high efficiency of installing three different functional groups in a single reaction, furnishing a wide array of polyfunctionalized aldehydes that are otherwise difficult to access with excellent functional group compatibility. Given its unique advantages, this method will be valuable in rapidly assembling complex molecules from readily available starting materials. More details are given in the article by Zhu et al. on page 1667–1673.
Back Cover
Back Cover
- Page: 1756
- First Published: 15 June 2022

The back cover picture shows a protecting-group-free palladium-catalyzed allylic coupling of cucurbitacin B, a natural product mainly found in cucurbitaceous plants. This convenient method provided a one-step approach to expand the chemical diversity of the C25 site of cucurbitacin B. Some of the compounds showed higher cytotoxicity against A549 cells than cucurbitacin B. More details are discussed in the article by Nan et al. on page 1662–1666.