Room-Temperature Reversible σ-Dimerization of a Phenalenyl Radical
Xue Dong
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorQuanchun Sun
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorZhongtao Feng
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorHuapeng Ruan
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorShuxuan Tang
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorMin Liu
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorYue Zhao
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorCorresponding Author
Yuanting Su
College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xinping Wang
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
E-mail: [email protected]; [email protected]Search for more papers by this authorXue Dong
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorQuanchun Sun
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorZhongtao Feng
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorHuapeng Ruan
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorShuxuan Tang
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorMin Liu
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorYue Zhao
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorCorresponding Author
Yuanting Su
College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xinping Wang
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, Jiangsu, 210023 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
Phenalenyl derivatives have been of tremendous interest due to their reversible dimerization in response to the external stimuli such as temperature. However, most of PLY derivatives only show the reversibility dependent on temperature. Herein, we report a unique PLY-based radical anionic salt with reversible σ-dimerization controlled by solvent rather than temperature. The structure and reversibility of this PLY anion system have been spectroscopically, crystallographically, and theoretically investigated. The molecule exists mainly as the σ-dimer form of K2[σ-12] in toluene, while it can be broken into the monomeric radical anion 1•– by re-dissolving in THF. Moreover, when THF is replaced with toluene, radical 1•– could be σ-dimerized back again. Theoretical calculations reveal that the potassium ions are necessary for the formation of the σ-dimer and the solvent polarity controls the σ-dimerization by regulating the presence of K+.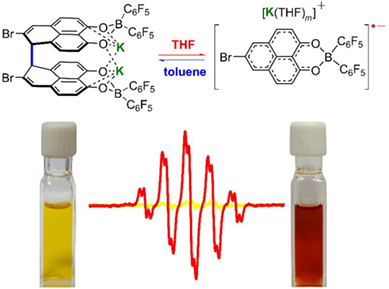
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200082-sup-0001-Supinfo.pdfPDF document, 1.5 MB |
Appendix S1 Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Sogo, P. B.; Nakazaki, M.; Calvin, M. Free radical from perinaphthene. J. Chem. Phys. 1957, 26, 1343–1345.
- 2(a) Hatanaka, K.; Morita, Y.; Ohba, T.; Yamaguchi, K.; Takui, T.; Kinoshita, M.; Nakasuji, K. Dimer formation and detection of neutral radical: 2,5-dimethyl-6-oxophenalenoxyl radical. Tetrahedron Lett. 1996, 37, 877–880;
(b) Goto, K.; Kubo, T.; Yamamoto, K.; Nakasuji, K.; Sato, K.; Shiomi, D.; Takui, T.; Kubota, M.; Kobayashi, T.; Yakusi, K.; Ouyang, J. A Stable neutral hydrocarbon radical: synthesis, crystal structure, and physical properties of 2,5,8-tri-tert-butyl-phenalenyl. J. Am. Chem. Soc. 1999, 121, 1619–1620;
(c) Morita, Y.; Ohba, T.; Haneda, N.; Maki, S.; Kawai, J.; Hatanaka, K.; Sato, K.; Shiomi, D.; Takui, T.; Nakasuji, K. New persistent radicals: synthesis and electronic spin structure of 2,5-di-tert-butyl-6-oxophenalenoxyl derivatives. J. Am. Chem. Soc. 2000, 122, 4825–4826;
(d) Koutentis, P. A.; Chen, Y.; Cao, Y.; Best, T. P.; Itkis, M. E.; Beer, L.; Oakley, R. T.; Cordes, A. W.; Brock, C. P.; Haddon, R. C. Perchlorophenalenyl radical. J. Am. Chem. Soc. 2001, 123, 3864–3871;
(e) Koutentis, P. A.; Haddon, R. C.; Oakley, R. T.; Cordes, A. W.; Brock, C. P. Perchlorophenalenyl radical, C13Cl9: a modulated structure with nine threefold-Symmetric molecules in the asymmetric unit. Acta Cryst. 2001, B57, 680–691;
(f) Morita, Y.; Aoki, T.; Fukui, K.; Nakazawa, S.; K. Tamaki, K.; Suzuki, S.; Fuyuhiro, A.; Yamamoto, K.; Sato, K.; Shiomi, D.; Naito, A.; Takui, T.; Nakasuji, K. A new trend in phenalenyl chemistry: a persistent neutral radical, 2,5,8-tri-tert-butyl-1,3-diazaphenalenyl, and the excited triplet state of the gable syn-dimer in the crystal of column motif. Angew. Chem. Int. Ed. 2002, 41, 1793–1796;
10.1002/1521-3773(20020517)41:10<1793::AID-ANIE1793>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar(g) Morita, Y.; Fukui, K.; Suzuki, S.; Aoki, T.; Nakazawa, S.; Tamaki, K.; Fuyuhiro, A.; Yamamoto, K.; Sato, K.; Shiomi, D.; Naito, A.; Takui, T.; Nakasuji, K. Electronic-spin and columnar crystal structures of stable 2,5,8-tri- tert-butyl-1,3-diazaphenalenyl radical. Polyhedron 2003, 22, 2199–2204; (h) Beer, L.; Mandal, S. K.; Reed, R. W.; Oakley, R. T.; Tham, F. S.; Donnadieu, B.; Haddon, R. C. The first electronically stabilized phenalenyl radical: effect of substituents on solution chemistry and solid-state structure. Cryst. Growth Des. 2007, 7, 802–809; (i) Hou, Y.; Wang, H.; Li, Z.; Liu, Y.; Wan, X.; Xue, X.; Chen, Y.; Yu, A. Organic radicals based on phenalenyl and verdazyl units. Tetrahedron Lett. 2011, 52, 3670–3673; (j) Nishida, S.; Kawai, J.; Moriguchi, M.; Ohba, T.; Haneda, N.; Fukui, K.; Fuyuhiro, A.; Shiomi, D.; Sato, K.; Takui, T.; Nakasuji, K.; Morita, Y. Control of exchange interactions in π dimers of 6-oxophenalenoxyl neutral π radicals: spin-density distributions and multicentered-two-electron bonding governed by topological symmetry and substitution at the 8-position. Chem. - Eur. J. 2013, 19, 11904–11915.
- 3(a) Sen, T. K.; Mukherjee, A.; Modak, A.; Ghorai, P. Kr.; Kratzert, D.; Granitzka, M.; Stalke D.; Mandal, S. K. Phenalenyl-based molecules: tuning the lowest unoccupied molecular orbital to design a catalyst. Chem. - Eur. J. 2012, 18, 54–58; (b) Ahmed, J.; Chakraborty, S.; Jose, A.; Sreejyothi, P.; Mandal, S. K. Integrating organic lewis acid and redox catalysis: the phenalenyl cation in dual role. J. Am. Chem. Soc. 2018, 140, 8330–8339.
- 4 Pariyar, A.; Vijaykumar, G.; Bhunia, M.; Dey, S. Kr.; Singh, S. K.; Kurungot, S.; Mandal, S. K. Switching Closed-Shell to Open-Shell Phenalenyl: Toward Designing Electroactive Materials. J. Am. Chem. Soc. 2015, 137, 5955–5960.
- 5(a) Nishida, S.; Morita, Y.; Fukui, K.; Sato, K.; Shiomi, D.; Takui, T.; Nakasuji, K. Spin transfer and solvato-/thermochromism induced by intramolecular electron transfer in a purely organic open-shell system. Angew. Chem. Int. Ed. 2005, 117, 7443–7446;
10.1002/ange.200502180 Google Scholar(b) Morita, Y.; Suzuki, S.; Fukui, K.; Nakazawa, S.; Kitagawa, H.; Kishida, H.; Okamoto, H.; Naito, A.; Sekine, A.; Ohashi, Y.; Shiro, M.; Sasaki, K.; Shiomi, D.; Sato, K.; Takui, T.; Nakasuji, K. Thermochromism in an organic crystal based on the coexistence of σ- and π-dimers. Nat Mater. 2008, 7, 48–51.
- 6(a) Lv, X.; Mao, J.; Liu, Y.; Huang, Y.; Ma, Y.; Yu, A.; Yin, S.; Chen, Y. A neutral stable and soluble polymer radical with low band gap and its photovoltaic application. Macromolecules 2008, 41, 501–503; (b) Wan, X.; Lv, X.; He, G.; Yu, A.; Chen, Y. Synthesis of neutral stable polyradicals and their application on photovoltaic devices. Eur. Polym. J. 2011, 47, 1018–1030.
- 7(a) Itkis, M. E.; Chi, X.; Cordes, A. W.; Haddon, R. C. Magneto-opto- electronic bistability in a phenalenyl-based neutral radical. Science 2002, 296, 1443-1445; (b) Pal, S. K.; Itki, M. E.; Tham, F. S.; Reed, R. W.; Oakley, R. T.; Haddon, R. C. Resonating valence-bond ground state in a phenalenyl-based neutral radical conductor. Science 2005, 309, 281–284; (c) Kubo, T.; Goto, Y.; Uruichi, M.; Yakushi, K.; Nakano, M.; Fuyuhiro, A.; Moritan, Y.; Nakasuji, K. Synthesis and characterization of acetylene-linked bisphenalenyl and metallic-like behavior in its charge-transfer complex. Chem. Asian J. 2007, 2, 1370–1379; (d) Bag, P.; Itkis, M. E.; Pal, S. K.; Bekyarova, E.; Donnadieu B.; Haddon, R. C. Synthesis, structure and solid state properties of cyclohexanemethylamine substituted phenalenyl based molecular conductor. Crystals 2012, 2, 446-465; (e) Wehrmann, C. M.; Charlton, R. T.; Chen, M. S. A concise synthetic strategy for accessing ambient stable bisphenalenyls toward achieving electroactive open-shell π-conjugated materials. J. Am. Chem. Soc. 2019, 141, 3240-3248; (f) Stekovic, D.; Itkis, M. E. Phenalenyl based neutral radical as a novel electrochromic material modulating visible to short-wave infrared light. RSC Adv. 2018, 8, 42068–42072; (g) Imran, M.; Wehrmann, C. M.; Chen, M. S. Open-Shell Effects on Optoelectronic Properties: Antiambipolar Charge Transport and Anti-Kasha Doublet Emission from a N-Substituted Bisphenalenyl. J. Am. Chem. Soc. 2020, 142, 38-43.
- 8(a) Morita, Y.; Nishida, S.; Kawai, J.; Fukui, K.; Nakazawa, S.; Sato, K.; Shiomi, D.; Takui, T.; Nakasuji, K. Redox-based spin diversity: a reversible topological spin switching in oxophenalenoxyl systems. Polyhedron 2003, 22, 2209–2213; (b) Morita, Y.; Nishida, S.; Kawai, J.; Takui, T.; Nakasuji, K. Oxophenalenoxyl: Novel stable neutral radicals with a unique spin-delocalized naturedepending on topological symmetries andredox states. Pure Appl. Chem. 2008, 80, 507–517; (c) Ahmed, J.; Datta, P.; Das, A.; Jomy, S.; Mandal, S. K. Switching between mono and doubly reduced odd alternant hydrocarbon: designing a redox catalyst. Chem. Sci. 2021, 12, 3039–3049; (d) Bhunia, M.; Sahoo, S. R.; Shaw, B. K.; Vaidya, S.; Pariyar, A.; Vijaykumar, G.; Adhikari, D.; Mandal, S. K. Storing redox equivalent in the phenalenyl backbone towards catalytic multi-electron reduction. Chem. Sci. 2019, 10, 7433–7441; (e) Ahmed, J.; Sreejyothi, P.; Vijaykumar, G.; Jose, A.; Raj, M.; Mandal, S. K. A new face of phenalenyl-based radicals in the transition metal-free C–H arylation of heteroarenes at room temperature: trapping the radical initiator via C–C σ-bond formation. Chem. Sci. 2017, 8, 7798–7806.
- 9 Wei, H.; Liu, Y.; Gopalakrishna, T. Y.; Phan, H.; Huang, X.; Guo, J.; Zhou, J.; Luo, S.; Wu J.; Zeng, Z. B−N−B bond embedded phenalenyl and its anions. J. Am. Chem. Soc. 2017, 139, 15760–15767.
- 10(a) Fujita W.; Awaga, K. Room-temperature magnetic bistability in organic radical crystals. Science 1999, 286, 261–262; (b) McManus, G. D.; Rawson, J. M.; Feeder, N.; van Duijn, J.; McInnes, E. J. L.; Novoa, J. J.; Burriel, R.; Palacio F.; Oliete, P. Synthesis, crystal structures, electronic structure and magnetic behaviour of the trithiatriazapentalenyl radical, C2S3N3. J. Mater. Chem. 2001, 11, 1992–2003; (c) Fujita, W.; Awaga, K.; Matsuzaki, H.; Okamoto, H. Room-temperature magnetic bistability in organic radical crystals: paramagnetic-diamagnetic phase transition in 1,3,5-trithia-2,4,6-triazapentalenyl. Phys. Rev. B 2002, 65, 064434; (d) Du, H.; Haddon, R. C.; Krossing, I.; Passmore, J.; Rawson J. M.; Schriver, M. J. Thermal hysteresis in dithiadiazolyl and dithiazolyl radicals induced by supercooling of paramagnetic liquids close to room temperature: a study of F3CC┌NSSN┐ and an interpretation of the behaviour of F3CC┌SNSC┐CF3. Chem. Commun. 2002, 17, 1836–1837; (e) Matsuzaki, H.; Fujita, W.; Awaga, K.; Okamoto, H. Photoinduced phase transition in an organic radical crystal with room-temperature optical and magnetic bistability. Phys. Rev. Lett. 2003, 91, 017403; (f) Brusso, J. L.; Clements, O. P.; Haddon, R. C.; Itkis, M. E.; Leitch, A. A.; Oakley, R. T.; Reed, R. W.; Richardson, J. F. Bistabilities in 1,3,2-dithiazolyl radicals. J. Am. Chem. Soc. 2004, 126, 8256-8265; (g) Brusso, J. L.; Clements, O. P.; Haddon, R. C.; Itkis, M. E.; Leitch, A. A.; Oakley, R. T.; Reed, R. W.; Richardson, J. F. Bistability and the Phase Transition in 1,3,2-Dithiazolo[4,5-b]pyrazin-2-yl. J. Am. Chem. Soc. 2004, 126, 14692–14693; (h) Lekin, K.; Winter, S. M.; Downie, L. E.; Bao, X.; Tse, J. S.; Desgreniers, S.; Secco, R. A.; Dube, P. A.; Oakley, R. T. Hysteretic spin crossover between a bisdithiazolyl radical and its hypervalent σ-dimer. J. Am. Chem. Soc. 2010, 132, 16212–16224; (i) Clarke, C. S.; Jornet-Somoza, J.; Mota, F.; Novoa, J. J.; Deumal, M. Origin of the magnetic bistability in molecule-based magnets: a first-principles bottom-up study of the TTTA crystal. J. Am. Chem. Soc. 2010, 132, 17817–17830; (j) Alberola, A.; Eisler, D. J.; Harvey, L.; Rawson, J. M. Molecular tailoring of spin-transition materials: preparation, crystal structure and magnetism of trifluoromethyl-pyridyl-1,3,2-dithiazolyl. CrystEngComm 2011, 13, 1794–1796; (k) Bates, D.; Robertson, C. M.; Leitch, A. A.; Dube, P. A.; Oakley, R. T. Magnetic bistability in naphtho-1,3,2-dithiazolyl: solid state interconversion of a thiazyl π-radical and its N−N σ-bonded dimer. J. Am. Chem. Soc. 2018, 140, 3846–3849; (l) Dragulescu-Andrasi, A.; Filatov, A. S.; Oakley, R. T.; Li, X.; Lekin, K.; Huq, A.; Pak, C.; Greer, S. M.; McKay, J.; Jo, M.; Lengyel, J.; Hung, I.; Maradzike, E.; DePrince, A. E.; Stoian, S. A.; Hill, S.; Hu, Y.-Y.; Shatruk, M. Radical dimerization in a plastic organic crystal leads to structural and magnetic bistability with wide thermal hysteresis. J. Am. Chem. Soc. 2019, 141, 17989–17994; (m) Taponen, A. I.; Ayadi, A.; Lahtinen, M. K.; Oyarzabal, I.; Bonhommeau, S.; Rouzières, M.; Mathonière, C.; Tuononen, H. M.; Clèrac, R.; Mailman, A. Room-temperature magnetic bistability in a salt of organic radical ions. J. Am. Chem. Soc. 2021, 143, 15912–15917.
- 11(a) Stuart, M. A. C.; Huck, W. T. S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G. B.; Szleifer, I.; Tsukruk, V. V.; Urban, M.; Winnik, F.; Zauscher, S.; Luzinov, I.; Minko, S. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113; (b) Jochum F. D.; Theato, P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483; (c) Jin, Y.; Yu, C.; Denman, R. J.; Zhang, W. Recent advances in dynamic covalent chemistry. Chem. Soc. Rev. 2013, 42, 6634–6654; (d) Peterson, J. P.; Winter, A. H. Solvent-Responsive Radical Dimers. Org. Lett. 2020, 22, 6072–6076.
- 12(a) Reid, D. H. Stable π-electron systems and new aromatic structures. Tetrahedron 1958, 3, 339-352; (b) Lü, J -M.; Rosokha S. V.; Kochi, J. K.; Stable (long-bonded) dimers via the quantitative self-association of different cationic, anionic, and ucharged π-radicals: structures, energetics, and optical transitions. J. Am. Chem. Soc. 2003, 125, 12161–12171; (c) Zheng, S.; Lan, J.; Khan, S. I.; Rubin, Y. Synthesis, characterization, and coordination chemistry of the 2-azaphenalenyl radical. J. Am. Chem. Soc. 2003, 125, 5786–5791; (d) Small, D.; Zaitsev, V.; Jung, Y.; Rosokha, S. V.; Head–Gordon M.; Kochi, J. K. Intermolecular π-to-π bonding between stacked aromatic dyads. experimental and theoretical binding energies and near-IR optical transitions for phenalenyl radical/radical versus radical/cation dimerizations. J. Am. Chem. Soc. 2004, 126, 13850–13858; (e) Liao, P.; Itkis, M. E.; Oakley, R. T.; Tham, F. S.; Haddon, R. C. Light-mediated C-C σ-bond driven crystallization of a phenalenyl radical dimer. J. Am. Chem. Soc. 2004, 126, 14297–14302; (f) Small, D.; Rosokha, S. V.; Kochi, J. K.; Head -Gordon, M. Characterizing the dimerizations of phenalenyl radicals by ab initio calculations and spectroscopy: σ-bond formation versus resonance π-stabilization. J. Phys. Chem. A 2005, 109, 11261–11267; (g) Zaitsev, V.; Rosokha, S. V.; Head- Gordon, M.; Kochi, J. K. Steric modulations in the reversible dimerizations of phenalenyl radicals via unusually weak carbon- centered π- and σ-bonds. J. Org. Chem. 2006, 71, 520–526; (h) Suzuki, S.; Morita, Y.; Fukui, K.; Sato, K.; Shiomi, D.; Takui T.; Nakasuji, K. Aromaticity on the pancake-bonded dimer of neutral phenalenyl radical as studied by MS and NMR spectroscopies and NICS analysis. J. Am. Chem. Soc. 2006, 128, 2530–2531; (i) Morita, Y.; Suzuki, S.; Sato, K.; Takui, T. Synthetic organic spin chemistry for structurally well-defined open-shell graphene fragments. Nat. Chem. 2011, 3, 197–204; (j) Mou, Z.; Uchida, K.; Kubo, T.; Kertesz, M. Evidence of σ- and π-dimerization in a series of phenalenyls. J. Am. Chem. Soc. 2014, 136, 18009–18022; (k) Uchida, K.; Hirao, Y.; Kurata, H.; Kubo, T.; Hatano, S.; Inoue, K. Dual association modes of the 2,5,8-tris(pentafluorophenyl)phenalenyl radical. Chem. Asian J. 2014, 9, 1823–1829; (l) Uchida, K.; Mou, Z.; Kertesz, M.; Kubo, T. Fluxional σ-bonds of the 2,5,8-trimethylphenalenyl dimer: direct observation of the sixfold σ-bond shift via a π-dimer. J. Am. Chem. Soc. 2016, 138, 4665–4672; (m) Kato, K.; Osuka, A. Platforms for stable carbon- centered radicals. Angew. Chem. Int. Ed. 2019, 58, 8978–8986; (n) Kubo, T. Synthesis, physical properties, and reactivity of stable, π-conjugated, carbon-centered radicals. Molecules 2019, 24, 665.
- 13 Morita, Y.; Nishida, S.; Fukui, K.; Hatanaka, K.; Ohba, T.; Sato, K.; Shiomi, D.; Takui, T.; Yamamoto, G.; Nakasuji, K. 2-Aryl substituted 3-oxophenalenoxyl radicals: π-spin structures and properties evaluated by dimer structure. Polyhedron 2005, 24, 2194–2199.
- 14(a) Parks, D. J.; Piers, W. E.; Yap, G. P. Synthesis, properties, and hydroboration activity of the highly electrophilic borane bis(pentafluorophenyl)borane, HB(C6F5)2. Organometallics 1998, 17, 5492–5503; (b) Feng, Z.; Chong, Y.; Tang, S.; Ruan, H.; Fang, Y.; Zhao, Y.; Jun, J.; Wang, X. Stable Boron-Containing Blue-Photoluminescent Radicals. Chin. J. Chem. 2021, 39, 1297–1302.
- 15 Bensch, L.; Gruber, I.; Janiak, C.; Müller, T. J. J. 5-(Hetero)aryl-substituted 9-hydroxyphenalenones: synthesis and electronic properties of multifunctional donor–acceptor conjugates. Chem. - Eur. J. 2017, 23, 10551–10558.
- 16Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, Jr. K.; Montgomery, J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16, Rev. C.01, Wallingford, CT, 2016.




