Copper-Catalyzed 1,2,5-Trifunctionalization of Terminal Alkynes Using SR as a Transient Directing Group for Radical Translocation
Jian Feng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Department of Chemistry, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
These authors contributed equally to this work.
Search for more papers by this authorFang Zhang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Department of Chemistry, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
These authors contributed equally to this work.
Search for more papers by this authorChenyun Shu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Department of Chemistry, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorCorresponding Author
Gangguo Zhu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Department of Chemistry, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
E-mail: [email protected]Search for more papers by this authorJian Feng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Department of Chemistry, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
These authors contributed equally to this work.
Search for more papers by this authorFang Zhang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Department of Chemistry, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
These authors contributed equally to this work.
Search for more papers by this authorChenyun Shu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Department of Chemistry, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorCorresponding Author
Gangguo Zhu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Department of Chemistry, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
The first Cu-catalyzed 1,2,5-trifunctionalization of abundant terminal alkynes is realized by merging hydrogen atom transfer and traceless directing strategy with SR as a transient group, delivering highly functionalized aldehydes in moderate to excellent yields with broad substrate scope. The synthetic utility of this method was demonstrated by the gram-scale reaction and downstream transformations of the resultant products. Given the high efficient installation of three different functional groups in a single reaction, it can serve as a very attractive method for rapidly assembling complex molecules from readily available starting materials.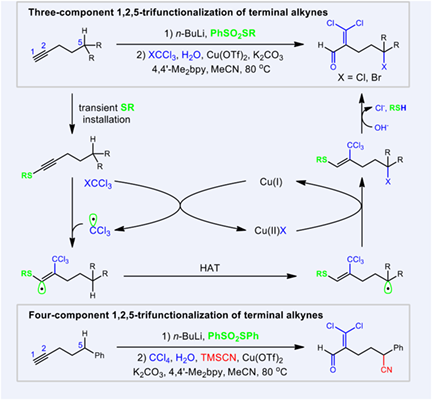
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200092-sup-0001-Supinfo.pdfPDF document, 6.2 MB |
Appendix S1 Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see: (a) Wille, U. Radical Cascades Initiated by Intermolecular Radical Addition to Alkynes and Related Triple Bond Systems. Chem. Rev. 2013, 113, 813−853; (b) Fang, G. C.; Bi, X. H. Silver-Catalysed Reactions of Alkynes: Recent Advances. Chem. Soc. Rev. 2015, 44, 8124−8173; (c) Huang, M.-H.; Hao, W.-J.; Li, G.; Tu, S.-J.; Jiang, B. Recent Advances in Radical Transformations of Internal Alkynes. Chem. Commun. 2018, 54, 10791−10811; (d) Ren, X.; Lu, Z. Visible Light Promoted Difunctionalization Reactions of Alkynes. Chin. J. Catal. 2019, 40, 1003−1019.
- 2For a recent review on trifunctionalization of alkynes, see: (a) Ghosh, S.; Lai, D.; Hajra, A. Trifunctionalization of Alkenes and Alkynes. Org. Biomol. Chem. 2020, 18, 7948−7976 and references cited therein.
- 3For selected reports on 1,1,2-trifunctionalization of alkynes, see: (a) Crick, P. J.; Simpkins, N. S.; Highton, A. Synthesis of the Asperparaline Core by a Radical Cascade. Org. Lett. 2011, 13, 6472−6475; (b) Huang, L.; Ye, L.; Li, X.-H.; Li, Z.-L.; Lin, J.-S.; Liu, X.-Y. Stereoselective Radical Cyclization Cascades Triggered by Addition of Diverse Radicals to Alkynes to Construct 6(5)–6–5 Fused Rings. Org. Lett. 2016, 18, 5284−5287; (c) Ni, S.; Sha, W.; Zhang, L.; Xie, C.; Mei, H.; Han, J.; Pan, Y. N-Iodosuccinimide-Promoted Cascade Trifunctionalization of Alkynoates: Access to 1,1-Diiodoalkenes. Org. Lett. 2016, 18, 712−715; (d) Sahoo, H.; Ramakrishna, I.; Mandal, A.; Baidya, M. Atom Transfer Oxidative Radical Cascade of Aryl Alkynoates towards 1,1-Dichalcogenide Olefins. Chem. Asian J. 2019, 14, 4549−4552; (e) Yan, J.; Cheo, H. W.; Teo, W. K.; Shi, X.; Wu, H.; Idres, S. B.; Deng, L.-W.; Wu, J. A Radical Smiles Rearrangement Promoted by Neutral Eosin Y as a Direct Hydrogen Atom Transfer Photocatalyst. J. Am. Chem. Soc. 2020, 142, 11357−11362; (f) Yang, Y.; Daniliuc, C. G.; Studer, A. 1,1,2-Trifunctionalization of Terminal Alkynes by Radical Addition–Translocation–Cyclization–Trapping for the Construction of Highly Substituted Cyclopentanes. Angew. Chem. Int. Ed. 2021, 60, 2145−2148; (g) Chalotra, N.; Shah, I. H.; Raheem, S.; Rizvi, M. A.; Shah, B. A. Visible-Light-Promoted Oxidative Annulation of Naphthols and Alkynes: Synthesis of Functionalized Naphthofurans. J. Org. Chem. 2021, 86, 16770−16784.
- 4 Gharpure, S. J.; Porwal, S. K. Topologically Driven Tandem Radical Cyclization-Based Strategy for the Synthesis of Oxa- and Aza-Cages. Tetrahedron Lett. 2009, 50, 7162−7165.
- 5For a 1,1,2,2-tetrafunctionalization of terminal alkynes, see: Tsuchii, K.; Doi, M.; Hirao, T.; Ogawa, A. Highly Selective Sequential Addition and Cyclization Reactions Involving Diphenyl Diselenide, an Alkyne, and Alkenes under Visible-Light Irradiation. Angew. Chem. Int. Ed. 2003, 42, 3490−3493.
- 6(a) Nie, X.; Cheng, C.; Zhu, G. Palladium-Catalyzed Remote Aryldifluoroalkylation of Alkenyl Aldehydes. Angew. Chem. Int. Ed. 2017, 56, 1898−1902; (b) Jin, W.; Zhou, Y.; Zhao, Y.; Ma, Q.; Kong, L.; Zhu, G. Nickel-Catalyzed Remote Arylation of Alkenyl Aldehydes Initiated by Radical Alkylation with Tertiary α-Carbonyl Alkyl Bromides. Org. Lett. 2018, 20, 1435−1438; (c) Shang, T.; Zhang, J.; Zhang, Y.; Zhang, F.; Li, X.-S.; Zhu, G. Photocatalytic Remote Oxyfluoroalkylation of Heteroalkynes: Regio-, Stereo-, and Site-Selective Access to Complex Fluoroalkylated (Z)-Alkenes. Org. Lett. 2020, 22, 3667−3672; (d) Zhou, Y.; Qin, Y.; Wang, Q.; Zhang, Z.; Zhu, G. Photocatalytic Sulfonylcarbocyclization of Alkynes Using SEt as a Traceless Directing Group: Access to Cyclopentenes and Indenes. Angew. Chem. Int. Ed. 2022, 61, e202110864.
- 7For recent reviews, see: (a) Li, W.; Xu, W.; Xie, J.; Yu, S.; Zhu, C. Distal Radical Migration Strategy: an Emerging Synthetic Means. Chem. Soc. Rev. 2018, 47, 654−667; (b) Stateman, L. M.; Nakafuku, K. M.; Nagib, D. A. Remote C–H Functionalization via Selective Hydrogen Atom Transfer. Synthesis 2018, 50, 1569−1586; (c) Sarkar, S.; Cheung, K. P. S.; Gevorgyan, V. C–H Functionalization Reactions Enabled by Hydrogen Atom Transfer to Carbon-Centered Radicals. Chem. Sci. 2020, 11, 12974−12993; (d) Chen, H.; Yu, S. Remote C–C Bond Formation via Visible Light Photoredox-Catalyzed Intramolecular Hydrogen Atom Transfer. Org. Biomol. Chem. 2020, 18, 4519−4532; (e) Guo, W.; Wang, Q.; Zhu, J. Visible Light Photoredox-Catalysed Remote C–H Functionalisation Enabled by 1,5-Hydrogen Atom Transfer (1,5-HAT). Chem. Soc. Rev. 2021, 50, 7359−7377; (f) Yue, B.; Wu, X.; Zhu, C. Recent Advances in Vinyl Radical-Mediated Hydrogen Atom Transfer. Chin. J. Org. Chem. 2022, 42, 458−470.
- 8For selected reports on 1,5-HAT of vinyl radicals, see: (a) Curran, D. P.; Shen, W. Radical Translocation Reactions of Vinyl radicals: Substituent Effects on 1,5-Hydrogen-Transfer Reactions. J. Am. Chem. Soc. 1993, 115, 6051−6059; (b) Hu, M.; Fan, J.-H.; Liu, Y.; Song, R.-J.; Ouyang, X.-H.; Li, J.-H. Metal-Free Radical [2+2+1] Carbocyclization of Benzene-Linked 1,n-Enynes: Dual C(sp3)–H Functionalization Adjacent to a Heteroatom. Angew. Chem. Int. Ed. 2015, 54, 9577−9580; (c) Qiu, J.-K.; Jiang, B.; Zhu, Y.-L.; Hao, W.-J.; Wang, D.-C.; Sun, J.; Wei, P.; Tu, S.-J.; Li, G. Catalytic Dual 1,1-H-Abstraction/Insertion for Domino Spirocyclizations. J. Am. Chem. Soc. 2015, 137, 8928−8931; (d) Gloor, C. S.; Dénès, F.; Renaud, P. Hydrosulfonylation Reaction with Arenesulfonyl Chlorides and Tetrahydrofuran: Conversion of Terminal Alkynes into Cyclopentylmethyl Sulfones. Angew. Chem. Int. Ed. 2017, 56, 13329−13332; (e) An, X.-D.; Jiao, Y.-Y.; Zhang, H.; Gao, Y.; Yu, S. Photoredox-Induced Radical Relay toward Functionalized β-Amino Alcohol Derivatives. Org. Lett. 2018, 20, 401−404; (f) Ratushnyy, M.; Parasram, M.; Wang, Y.; Gevorgyan, V. Palladium-Catalyzed Atom- Transfer Radical Cyclization at Remote Unactivated C(sp3)−H Sites: Hydrogen-Atom Transfer of Hybrid Vinyl Palladium Radical Intermediates. Angew. Chem. Int. Ed. 2018, 57, 2712−2715; (g) Wu, S.; Wu, X.; Wang, D.; Zhu, C. Regioselective Vinylation of Remote Unactivated C(sp3)−H Bonds: Access to Complex Fluoroalkylated Alkenes. Angew. Chem. Int. Ed. 2019, 58, 1499−1503; (h) Wu, S.; Wu, X.; Wu, Z.; Zhu, C. Regioselective Introduction of Vinyl Trifluoromethylthioether to Remote Unactivated C(sp3)−H bonds via Radical Translocation Cascade. Sci. China Chem. 2019, 62, 1507−1511; (i) Liu, T.; Qu, C.; Xie, J.; Zhu, C. Photoinduced Atom-Economical Iterative Hydrotrifluoromethylation of Terminal Alkynes and Remote C(sp3)−H Functionalization. Chin. J. Org. Chem. 2019, 39, 1613−1622; (j) Wan, Y.; Shang, T.; Lu, Z.; Zhu, G. Photocatalytic 1,1-Hydrofluoroalkylation of Alkynes with a Concurrent Vicinal Acylation: An Access to Fluoroalkylated Cyclic Ketones. Org. Lett. 2019, 21, 4187−4191; (k) Shu, C.; Feng, J.; Zheng, H.; Cheng, C.; Yuan, Z.; Zhang, Z.; Xue, X.-S.; Zhu, G. Internal Alkyne-Directed Fluorination of Unactivated C(sp3)−H Bonds. Org. Lett. 2020, 22, 9398−9403; (l) Xiong, Z.; Zhang, F.; Yu, Y.; Tan, Z.; Zhu, G. AIBN-Induced Remote Trifluoromethyl-Alkynylation of Thioalkynes. Org. Lett. 2020, 22, 4088−4092; (m) Li, H.; Guo, L.; Feng, X.; Huo, L.; Zhu, S.; Chu, L. Sequential C–O Decarboxylative Vinylation/C–H Arylation of Cyclic Oxalates via a Nickel-Catalyzed Multicomponent Radical Cascade. Chem. Sci. 2020, 11, 4904−4910; (n) Xie, S.; Li, Y.; Liu, P.; Sun, P. Visible Light-Induced Radical Addition/Annulation to Construct Phenylsulfonyl-Functionalized Dihydrobenzofurans Involving an Intramolecular 1,5-Hydrogen Atom Transfer Process. Org. Lett. 2020, 22, 8774−8779; (o) Zhu, H.; Shang, T.; Lu, Z.; Luo, F.; Zhu, G. Visible-Light Photocatalytic Remote Halo-Difluoroalkylation of Thioalkynes. Chin. J. Org. Chem. 2020, 40, 3410−3419; (p) Zhong, L.-J.; Li, Y.; An, D.-L.; Li, J.-H. Heteroannulation of N-Fluoro-N-alkylsulfonamides with Terminal Alkynes via Remote C(sp3)–H Functionalization. ACS Catal. 2021, 11, 383−389.
- 9 Kharasch, M. S.; Jenson, E. V.; Urry, W. H. Addition of Carbon Tetrachloride and Chloroform to Olefins. Science 1945, 102, 128−130.
- 10For selected reports on Cu-catalyzed radical trichloromethylation using CCl4, see: (a) Liu, Z.; Chen, H.; Lv, Y.; Tan, X.; Shen, H.; Yu, H.-Z.; Li, C. Radical Carbofluorination of Unactivated Alkenes with Fluoride Ions. J. Am. Chem. Soc. 2018, 140, 6169−6175; (b) Balili, M. N. C.; Pintauer, T. Photoinitiated Ambient Temperature Copper-Catalyzed Atom Transfer Radical Addition (ATRA) and Cyclization (ATRC) Reactions in the Presence of Free-Radical Diazo Initiator (AIBN). Dalton Trans. 2011, 40, 3060−3066; (c) Su, Y.-L.; Tram, L.; Wherritt, D.; Arman, H.; Griffith, W. P.; Doyle, M. P. α-Amino Radical-Mediated Diverse Difunctionalization of Alkenes: Construction of C−C, C−N, and C−S Bonds. ACS Catal. 2020, 10, 13682–13687.
- 11 Barnett, R. E.; Jencks, W. P. Diffusion-Controlled and Concerted Base Catalysis in the Decomposition of Hemithioacetals. J. Am. Chem. Soc. 1969, 91, 6758−6765.
- 12For recent reviews on polychloroalkylation, see: (a) Liang, Y.-Y.; Lv, G.-F.; Ouyang, X.-H.; Song, R.-J.; Li, J.-H. Recent Developments in the Polychloroalkylation by Use of Simple Alkyl Chlorides. Adv. Synth. Catal. 2021, 363, 290−304; (b) Huang, G.; Yu, J.-T.; Pan, C. Recent Advances in Polychloromethylation Reactions. Adv. Synth. Catal. 2021, 363, 305−327; For selected reports on trichloromethylation of alkynes, see: (c) Wu, D.; Hao, W.-J.; Rao, Q.; Lu, Y.; Tu, S.-J.; Jiang, B. Engaging 1,7-Diynes in a Photocatalytic Kharasch-Type Addition/ 1,5-(SN″)-Substitution Cascade toward β-gem-Dihalovinyl Carbonyls. Chem. Commun. 2021, 57, 1911−1914; (d) Wang, L.; Xu, T.; Rao, Q.; Zhang, T.-S.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Photocatalytic Biheterocyclization of 1,7-Diynes for Accessing Skeletally Diverse Tricyclic 2-Pyranones. Org. Lett. 2021, 23, 7845−7850; (e) Geng, F.; Wang, S.; Song, K.; Hao, W.; Jiang, B. Visible-Light-Driven Photocatalytic Kharasch-Type Addition of 1,6-Enynes. Chin. J. Org. Chem. 2021, 41, 4815−4824; (f) Heiba, E. I. Dessau, R. M. Free-Radical Isomerization. I. A Novel Rearrangement of Vinyl Radicals. J. Am. Chem. Soc. 1967, 89, 3772−3777; For selected reports on trichloromethylation of alkenes, see: (g) Beaumont, S.; Ilardi, E. A.; Monroe, L. R.; Zakarian, A. Valence Tautomerism in Titanium Enolates: Catalytic Radical Haloalkylation and Application in the Total Synthesis of Neodysidenin. J. Am. Chem. Soc. 2010, 132, 1482–1483; (h) Lu, M.-Z.; Loh, T.-P. Iron-Catalyzed Cascade Carbochloromethylation of Activated Alkenes: Highly Efficient Access to Chloro-Containing Oxindoles. Org. Lett. 2014, 16, 4698−4701; (i) Ueda, M.; Doi, N.; Miyagawa, H.; Sugita, S.; Takeda, N.; Shinada, T.; Miyata, O. Reaction of Cyclopropenes with a Trichloromethyl Radical: Unprecedented Ring-Opening Reaction of Cyclopropanes with Migration. Chem. Commun. 2015, 51, 4204−4207; (j) Chen, B.; Fang, C.; Liu, P.; Ready, J. M. Rhodium-Catalyzed Enantioselective Radical Addition of CX4 Reagents to Olefins. Angew. Chem. Int. Ed. 2017, 56, 8780–8784; (k) Roslan, I. I.; Zhang, H.; Ng, K.-H.; Jaenicke, S.; Chuah, G.-K. A Visible Light and Iron-mediated Carbocationic Route to Polysubstituted 1-Halonaphthalenes by Benzannulation using Allylbenzenes and Polyhalomethanes. Adv. Synth. Catal. 2021, 363, 1007−1013; (l) Kusakabe, M.; Nagao, K.; Ohmiya, H. Radical Relay Trichloromethylacylation of Alkenes through N-Heterocyclic Carbene Catalysis. Org. Lett. 2021, 23, 7242−7247; (m) Liu, Y.; Zhang, J.-L.; Song, R.-J.; Li, J.-H. 1,2-Alkylarylation of Activated Alkenes with Dual C–H Bonds of Arenes and Alkyl Halides toward Polyhalo-Substituted Oxindoles. Org. Chem. Front. 2014, 1, 1289−1294; (n) Huang, J.; Liang, Y.-Y.; Ouyang, X.-H.; Xiao, Y.-T.; Qin, J.-H.; Song, R.-J.; Li, J.-H. Org. Chem. Front. 2021, 8, 7009−7014; (o) Liang, Y.-Y.; Huang, J.; Ouyang, X.-H.; Qin, J.-H.; Song, R.-J.; Li, J.-H. Radical-Mediated Alkoxypolyhaloalkylation of Styrenes with Polyhaloalkanes and Alcohols via C(sp3)-H Bond Cleavage. Chem. Commun. 2021, 57, 3684−3687.
- 13For selected reports, see: (a) Voica, A.-F.; Mendoza, A.; Gutekunst, W. R.; Fraga, J. O.; Baran, P. S. Guided desaturation of unactivated aliphatics. Nat. Chem. 2012, 4, 629–635; (b) Parasram, M.; Chuentragool, P.; Sarkar, D.; Gevorgyan, V. Photoinduced Formation of Hybrid Aryl Pd-Radical Species Capable of 1,5-HAT: Selective Catalytic Oxidation of Silyl Ethers into Silyl Enol Ethers. J. Am. Chem. Soc. 2016, 138, 6340−6343; (c) Jin, W.; Yu, S. Photoinduced and Palladium-Catalyzed Remote Desaturation of Amide Derivatives. Org. Lett. 2021, 23, 6931−6935; (d) Xia, Y.; Jana, K.; Studer, A. Remote Radical Desaturation of Unactivated C-H Bonds in Amides. Chem. - Eur. J. 2021, 27, 16621−16625; (e) Stateman, L. M.; Dare, R. M.; Paneque, A. N.; Nagib, D. A. Aza-heterocycles via Copper-Catalyzed, Remote C-H Desaturation of Amines. Chem 2022, 8, 210−224 and references cited therein.
- 14For selected reports, see: (a) Zhang, Z.; Zhang, X.; Nagib, D. A. Chiral Piperidines from Acyclic Amines via Enantioselective, Radical-Mediated δ C–H Cyanation. Chem 2019, 5, 3127–3134; (b) Cheng, Z.; Chen, P.; Liu, G. Enantioselective Cyanation of Remote C—H Bonds via Cooperative Photoredox and Copper Catalysis. Acta Chim. Sinica 2019, 77, 856−860; (c) Wang, C.-Y.; Qin, Z.-Y.; Huang, Y.-L.; Jin, R.-X.; Lan, Q.; Wang, X.-S. Enantioselective Copper-Catalyzed Cyanation of Remote C(sp3)-H Bonds Enabled by 1,5-Hydrogen Atom Transfer. iScience 2019, 21, 490–498; (d) Chen, H.; Jin, W.; Yu, S. Enantioselective Remote C(sp3)−H Cyanation via Dual Photoredox and Copper Catalysis. Org. Lett. 2020, 22, 5910−5914 and references cited therein.




