Electrochemical Synthesis of Sulfonyl Fluorides with Triethylamine Hydrofluoride
Lei Zhang
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Xu Cheng
Institute of Chemistry and Biomedical Sciences, Jiangsu Key Laboratory of Advanced Organic Materials, National Demon-stration Center for Experimental Chemistry Education, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Qi-Lin Zhou
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]; [email protected]Search for more papers by this authorLei Zhang
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Xu Cheng
Institute of Chemistry and Biomedical Sciences, Jiangsu Key Laboratory of Advanced Organic Materials, National Demon-stration Center for Experimental Chemistry Education, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Qi-Lin Zhou
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
Hydrofluoride is an industry-preferred fluoride source, and finds extensively application to manufacture diverse fluoro chemicals. The Et3N-3HF complex is a liquid HF with improve safety. In this work, we report electrochemical synthesis of a series of sulfonyl fluoride with Et3N-3HF as fluoride source. The sulfinic salt is a smell-less, non-volatile, and air-stable sulfur source in this reaction. With the combination of Et3N-3HF and aryl/alkyl sulfinic salt, the sulfonyl fluorides are achieved without the use of external oxidant. In addition, we demonstrate further advantage in a tandem reaction involving Pd-catalyzed C—S cross-coupling and formation of S—F bond. A variety of functional groups including amino acids, heterocycles, halides are well tolerated.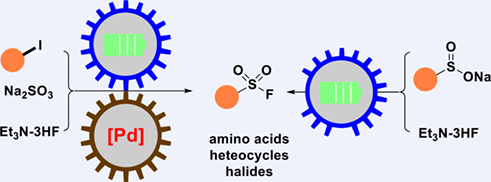
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200112-sup-0001-Supinfo.pdfPDF document, 11.8 MB |
Appendix S1 Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Dong, J.; Krasnova, L.; Finn, M. G.; Sharpless, K. B. Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angew. Chem. Int. Ed. 2014, 53, 9430–9448; (b) Gembus, V.; Marsais, F.; Levacher, V. An Efficient Organocatalyzed Interconversion of Silyl Ethers to Tosylates Using DBU and p-Toluenesulfonyl Fluoride. Synlett 2008, 2008, 1463–1466.
- 2(a) Ball, N. D. Properties and Applications of Sulfur(VI) Fluorides. In Emerging Fluorinated Motifs: Synthesis, Properties, and Applications, Wiley-VCH Verlag GmbH & Co. KGaA, 2020, pp. 621–674; (b) Chelagha, A.; Louvel, D.; Taponard, A.; Berthelon, R.; Tlili, A. Synthetic Routes to Arylsulfonyl Fluorides. Catalysts 2021, 11, 830; (c) Zhong, T.; Chen, Z.; Yi, J.; Lu, G.; Weng, J. Recent progress in the synthesis of sulfonyl fluorides for SuFEx click chemistry. Chin. Chem. Lett. 2021, 32, 2736–2750.
- 3 Mukherjee, P.; Woroch, C. P.; Cleary, L.; Rusznak, M.; Franzese, R. W.; Reese, M. R.; Tucker, J. W.; Humphrey, J. M.; Etuk, S. M.; Kwan, S. C.; am Ende, C. W.; Ball, N. D. Sulfonamide Synthesis via Calcium Triflimide Activation of Sulfonyl Fluorides. Org. Lett. 2018, 20, 3943–3947.
- 4(a) Yin, J.; Zarkowsky, D. S.; Thomas, D. W.; Zhao, M. M.; Huffman, M. A. Direct and Convenient Conversion of Alcohols to Fluorides. Org. Lett. 2004, 6, 1465–1468; (b) Nielsen, M. K.; Ugaz, C. R.; Li, W.; Doyle, A. G. PyFluor: A Low-Cost, Stable, and Selective Deoxyfluorination Reagent. J. Am. Chem. Soc. 2015, 137, 9571–9574; (c) Nielsen, M. K.; Ahneman, D. T.; Riera, O.; Doyle, A. G. Deoxyfluorination with Sulfonyl Fluorides: Navigating Reaction Space with Machine Learning. J. Am. Chem. Soc. 2018, 140, 5004–5008.
- 5(a) Berg, S.; Bergh, M.; Hellberg, S.; Högdin, K.; Lo-Alfredsson, Y.; Söderman, P.; von Berg, S.; Weigelt, T.; Ormö, M.; Xue, Y.; Tucker, J.; Neelissen, J.; Jerning, E.; Nilsson, Y.; Bhat, R. Discovery of Novel Potent and Highly Selective Glycogen Synthase Kinase-3β (GSK3β) Inhibitors for Alzheimer's Disease: Design, Synthesis, and Characterization of Pyrazines. J. Med. Chem. 2012, 55, 9107–9119; (b) Mukherjee, H.; Debreczeni, J.; Breed, J.; Tentarelli, S.; Aquila, B.; Dowling, J. E.; Whitty, A.; Grimster, N. P. A study of the reactivity of S(VI)–F containing warheads with nucleophilic amino-acid side chains under physiological conditions. Org. Biomol. Chem. 2017, 15, 9685–9695.
- 6 Wang, H.; Zhou, F.; Ren, G.; Zheng, Q.; Chen, H.; Gao, B.; Klivansky, L.; Liu, Y.; Wu, B.; Xu, Q.; Lu, J.; Sharpless, K. B.; Wu, P. SuFEx-Based Polysulfonate Formation from Ethenesulfonyl Fluoride–Amine Adducts. Angew. Chem. Int. Ed. 2017, 56, 11203–11208.
- 7(a) Hett, E. C.; Xu, H.; Geoghegan, K. F.; Gopalsamy, A.; Kyne, R. E.; Menard, C. A.; Narayanan, A.; Parikh, M. D.; Liu, S.; Roberts, L.; Robinson, R. P.; Tones, M. A.; Jones, L. H. Rational Targeting of Active-Site Tyrosine Residues Using Sulfonyl Fluoride Probes. ACS Chem. Biol. 2015, 10, 1094–1098; (b) Narayanan, A.; Jones, L. H. Sulfonyl fluorides as privileged warheads in chemical biology. Chem. Sci. 2015, 6, 2650–2659; (c) Fadeyi, O.; Parikh, M. D.; Chen, M. Z.; Kyne Jr., R. E.; Taylor, A. P.; O'Doherty, I.; Kaiser, S. E.; Barbas, S.; Niessen, S.; Shi, M.; Weinrich, S. L.; Kath, J. C.; Jones, L. H.; Robinson, R. P. Chemoselective Preparation of Clickable Aryl Sulfonyl Fluoride Monomers: A Toolbox of Highly Functionalized Intermediates for Chemical Biology Probe Synthesis. ChemBioChem 2016, 17, 1925–1930.
- 8(a) Brouwer, A. J.; Jonker, A.; Werkhoven, P.; Kuo, E.; Li, N.; Gallastegui, N.; Kemmink, J.; Florea, B. I.; Groll, M.; Overkleeft, H. S.; Liskamp, R. M. J. Peptido Sulfonyl Fluorides as New Powerful Proteasome Inhibitors. J. Med. Chem. 2012, 55, 10995–11003; (b) Dubiella, C.; Cui, H.; Gersch, M.; Brouwer, A. J.; Sieber, S. A.; Krüger, A.; Liskamp, R. M. J.; Groll, M. Selective Inhibition of the Immunoproteasome by Ligand-Induced Crosslinking of the Active Site. Angew. Chem. Int. Ed. 2014, 53, 11969–11973; (c) Herrero Alvarez, N.; van de Langemheen, H.; Brouwer, A. J.; Liskamp, R. M. J. Potential peptidic proteasome inhibitors by incorporation of an electrophilic trap based on amino acid derived α-substituted sulfonyl fluorides. Biorg. Med. Chem. 2017, 25, 5055–5063.
- 9(a) Davies, W.; Dick, J. H. CCLXXXVI.—Aromatic sulphonyl fluorides. A convenient method of preparation. J. Chem. Soc. (Resumed) 1931, 2104–2109;
10.1039/JR9310002104 Google Scholar(b) Bianchi, T. A.; Cate, L. A. Phase transfer catalysis. Preparation of aliphatic and aromatic sulfonyl fluorides. J. Org. Chem. 1977, 42, 2031–2032; (c) Talko, A.; Barbasiewicz, M. Nucleophilic Fluorination with Aqueous Bifluoride Solution: Effect of the Phase- Transfer Catalyst. ACS Sustainable Chem. Eng. 2018, 6, 6693–6701.
- 10 Pérez-Palau, M.; Cornella, J. Synthesis of Sulfonyl Fluorides from Sulfonamides. Eur. J. Org. Chem. 2020, 2020, 2497–2500.
- 11 Tang, L.; Yang, Y.; Wen, L.; Yang, X.; Wang, Z. Catalyst-free radical fluorination of sulfonyl hydrazides in water. Green Chem. 2016, 18, 1224–1228.
- 12 Liu, Y.; Yu, D.; Guo, Y.; Xiao, J. C.; Chen, Q. Y.; Liu, C. Arenesulfonyl Fluoride Synthesis via Copper-Catalyzed Fluorosulfonylation of Arenediazonium Salts. Org. Lett. 2020, 22, 2281–2286.
- 13(a) Bui, T. T.; Tran, V. H.; Kim, H. K. Visible-Light-Mediated Synthesis of Sulfonyl Fluorides from Arylazo Sulfones. Adv. Synth. Catal. 2022, 364, 341–347; (b) Lin, Q.; Ma, Z.; Zheng, C.; Hu, X. J.; Guo, Y.; Chen, Q. Y.; Liu, C. Arenesulfonyl Fluoride Synthesis via Copper-free Sandmeyer-type Fluorosulfonylation of Arenediazonium Salts. Chin. J. Chem. 2020, 38, 1107–1110; (c) Liu, S.; Huang, Y.; Xu, X. H.; Qing, F. L. Fluorosulfonylation of Arenediazonium Tetrafluoroborates with Na2S2O5 and N-fluorobenzenesulfonimide. J. Fluorine Chem. 2020, 240, 109653; (d) Pan, Q.; Liu, Y.; Pang, W.; Wu, J.; Ma, X.; Hu, X.; Guo, Y.; Chen, Q. Y.; Liu, C. Copper-catalyzed Three-component Reaction of Arylhydrazine Hydrochloride, DABSO, and NFSI for the Synthesis of Arenesulfonyl Fluorides. Org. Biomol. Chem. 2021, 19, 8999–9003.
- 14(a) Zhong, T.; Pang, M. K.; Chen, Z. D.; Zhang, B.; Weng, J.; Lu, G. Copper-free Sandmeyer-type Reaction for the Synthesis of Sulfonyl Fluorides. Org. Lett. 2020, 22, 3072–3078; (b) Magre, M.; Cornella, J. Redox-Neutral Organometallic Elementary Steps at Bismuth: Catalytic Synthesis of Aryl Sulfonyl Fluorides. J. Am. Chem. Soc. 2021, 143, 21497–21502.
- 15 Wang, L.; Cornella, J. A Unified Strategy for Arylsulfur(VI) Fluorides from Aryl Halides: Access to Ar-SOF3 Compounds. Angew. Chem. Int. Ed. 2020, 59, 23510–23515.
- 16(a) Kwon, J.; Kim, B. M. Synthesis of Arenesulfonyl Fluorides via Sulfuryl Fluoride Incorporation from Arynes. Org. Lett. 2019, 21, 428–433; (b) Lee, C.; Ball, N. D.; Sammis, G. M. One-pot fluorosulfurylation of Grignard reagents using sulfuryl fluoride. Chem. Commun. 2019, 55, 14753–14756.
- 17(a) Nie, X.; Xu, T.; Song, J.; Devaraj, A.; Zhang, B.; Chen, Y.; Liao, S. Radical Fluorosulfonylation: Accessing Alkenyl Sulfonyl Fluorides from Alkenes. Angew. Chem. Int. Ed. 2021, 60, 3956–3960; (b) Nie, X.; Xu, T.; Hong, Y.; Zhang, H.; Mao, C.; Liao, S. Introducing A New Class of Sulfonyl Fluoride Hubs via Radical Chloro-Fluorosulfonylation of Alkynes. Angew. Chem. Int. Ed. 2021, 60, 22035–22042; (c) Chen, D.; Nie, X.; Feng, Q.; Zhang, Y.; Wang, Y.; Wang, Q.; Huang, L.; Huang, S.; Liao, S. Electrochemical Oxo-Fluorosulfonylation of Alkynes under Air: Facile Access to β-Keto Sulfonyl Fluorides. Angew. Chem. Int. Ed. 2021, 60, 27271–27276.
- 18(a) Yan, M.; Kawamata, Y.; Baran, P. S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319; (b) Wiebe, A.; Gieshoff, T.; Mohle, S.; Rodrigo, E.; Zirbes, M.; Waldvogel, S. R. Electrifying Organic Synthesis. Angew. Chem. Int. Ed. 2018, 57, 5594–5619; (c) Pollok, D.; Waldvogel, S. R. Electro-organic synthesis - a 21(st) century technique. Chem. Sci. 2020, 11, 12386–12400.
- 19(a) Laudadio, G.; Bartolomeu, A. d. A.; Verwijlen, L. M. H. M.; Cao, Y.; de Oliveira, K. T.; Noël, T. Sulfonyl Fluoride Synthesis through Electrochemical Oxidative Coupling of Thiols and Potassium Fluoride. J. Am. Chem. Soc. 2019, 141, 11832–11836; (b) Cao, Y.; Adriaenssens, B.; de Bartolomeu, A.; Laudadio, G.; de Oliveira, K. T.; Noël, T. Accelerating sulfonyl fluoride synthesis through electrochemical oxidative coupling of thiols and potassium fluoride in flow. J. Flow Chem. 2020, 10, 191–197.
- 20 Dai, J. X.; Wang, Y. P.; Zhao, S. Y. Advances in the Application of Triethylamine Tris(hydrofluoride) to Organic Synthesis. Chin. J. Org. Chem. 2009, 29, 1307–1316.
- 21(a) Wang, Z.; Yang, S.; Chen, W.; Luo, X.; Luo, S.; Zhou, Y.; Wang, B. Research Progress in C-S Bond Formation Reaction of Olefins with Organic Sulfur Reagents under Photocatalyst-Free and Non-Electrochemical Conditions. Chin. J. Org. Chem. 2021, 41, 171–184; (b) Wang, T.; Huang, J.; Shen, Q.; Zhang, J.; Xiong, Y. Electrochemical Coupling of the Sulfonic Acid Sodium and Tertiary Amines for the Synthesis of β-Amidovinyl Sulfones. Chin. J. Org. Chem. 2021, 41, 2735–2742.
- 22For some reviews on electrochemical dehydrogenative coupling, see: (a) Ye, Z.; Zhang, F. Recent Advances in Constructing Nitrogen-Containing Heterocycles via Electrochemical Dehydrogenation. Chin. J. Chem. 2019, 37, 513–528;
(b) Yuan, Y.; Lei, A. Electrochemical Oxidative Cross-Coupling with Hydrogen Evolution Reactions. Acc. Chem. Res. 2019, 52, 3309–3324;
(c) Rockl, J. L.; Pollok, D.; Franke, R.; Waldvogel, S. R. A Decade of Electrochemical Dehydrogenative C, C-Coupling of Aryls. Acc. Chem. Res. 2020, 53, 45–61;
(d) Wang, P.; Gao, X. L.; Huang, P. F.; Lei, A. Recent Advances in Electrochemical Oxidative Cross-Coupling of Alkenes with H2 Evolution. ChemCatChem 2020, 12, 27–40;
(e) Ma, C.; Fang, P.; Liu, Z.-R.; Xu, S.-S.; Xu, K.; Cheng, X.; Lei, A.; Xu, H.-C.; Zeng, C.; Mei, T.-S. Recent advances in organic electrosynthesis employing transition metal complexes as electrocatalysts. Sci. Bull. 2021, 66, 2412–2429.;
(f) Chen, N.; Xu, H.-C. Electrochemical generation of nitrogen-centered radicals for organic synthesis. Green Synth. Catal. 2021, 2, 165–178;
10.1016/j.gresc.2021.03.002 Google Scholar(g) Cheng, X.; Lei, A.; Mei, T.; Xu, H.; Xu, K.; Zeng, C. Recent Applications of Homogeneous Catalysis in Electrochemical Organic Synthesis. CCS Chem. 2022, 4, 1120–1152.




