Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture: L-Lysine/imidazole-catalyzed Multicomponent Cascade Reaction: Facile Synthesis of C5-substituted 3-Methylcyclohex-2-enones (Chin. J. Chem. 8/2013)
- Page: 973
- First Published: 15 August 2013

The cover picture shows a facile and green approach for the direct synthesis of C5-substituted 3-methylcyclohex-2-enones via the Aldol-Robinson cascade reactions. Lysine is the active center of type-I aldolase and could effectively catalyze the aldol reaction between aldehydes and acetones. Inspired by the catalytic mechanism of type-I aldolase, L-lysine/imidazole catalytic system was designed to catalyze the cascade reaction to produce a series of C5-substituted 3-methylcyclohex-2- enones with a versatile aldehyde group with the yields of 16% –92%. The use of simple and readily available materials, mild reaction conditions, simple execution, good yields, and wide synthetic potential of the products, make this new catalytic system attractive for academic research and practical applications. More details are discussed in the article by Lin et al. on page 997–1002.
Contents
Communications
Solvent-free Synthesis of Hexagonal Iron Sulfide Nanoflowers
- Pages: 983-986
- First Published: 17 June 2013
Selective Reduction of Nitroarenes with Molybdenum Disulfide
- Pages: 987-991
- First Published: 05 July 2013
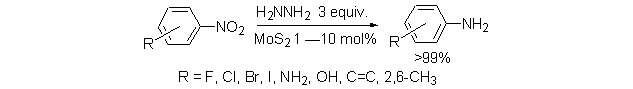
MoS2 was used as a catalyst for the reduction of nitrobenzene under mild conditions. Nitroarenes with halides (F, Cl, Br and I) were reduced selectively without dehaloganation, and functional groups such as NH2, OH, alkene groups were tolerated during the reduction of the nitro compounds. The reduction of p-chloronitrobenzene was studied over MoS2 and Pd/C respectively with hydrazine. p-Chloroaniline was obtained quantitively with MoS2, but some aniline formed by dehaloganation with Pd/C.
Full Papers
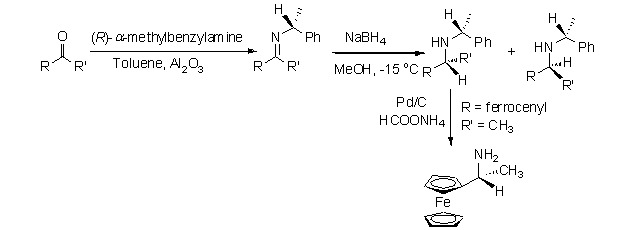
A Highly Efficient Synthesis of Optically Active Ferrocenylethylamines via Hydride Reduction of Chiral Ferrocenylketimines
- Pages: 992-996
- First Published: 05 July 2013
L-Lysine/imidazole-catalyzed Multicomponent Cascade Reaction: Facile Synthesis of C5-substituted 3-Methylcyclohex-2-enones
- Pages: 997-1002
- First Published: 19 July 2013
On Water: Free Catalysis Approach for the Synthesis of Nitrones from Hydroxamic Acids and Dimethy/Diethyl Acetylenedicarboxylate
- Pages: 1003-1006
- First Published: 19 July 2013
Copper(I)-Catalyzed Intramolecular Direct C-Arylation of Azoles with Aryl Bromides
- Pages: 1007-1010
- First Published: 19 July 2013
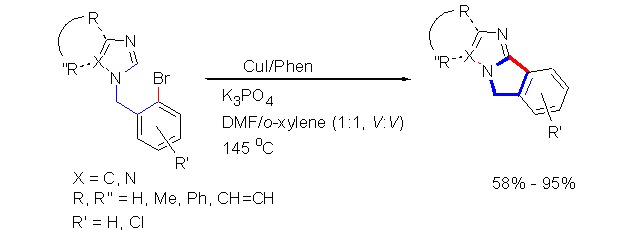
A concise route to access 5H-imidazo[2,1-a]isoindole heterofused compounds by copper(I)-catalyzed intramolecular coupling of non-activated aryl bromides with azoles is reported for the first time. With CuI as catalyst, 1,10-phenanthroline as ligand, and K3PO4 as base, the reactions of 1-(2-bromobenzyl)-1H-imidazoles in DMF/o-xylene (1:1, V:V) at 145°C afford the corresponding substituted 5H-imidazo[2,1-a]isoindoles in high yields via intramolecular C-arylation.
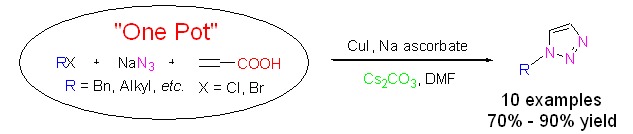
Easy One-Pot Synthesis of 1-Monosubstituted Aliphatic 1,2,3-Triazoles from Aliphatic Halides, Sodium Azide and Propiolic Acid by a Click Cycloaddition/Decarboxylation Process
- Pages: 1011-1014
- First Published: 29 May 2013
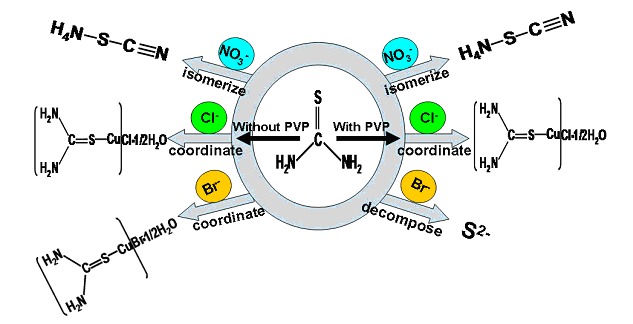
Phase Evolution of CuS System in Ethylene Glycol Solution: the Effect of Anion and PVP on the Transformation of Thiourea
- Pages: 1015-1021
- First Published: 19 July 2013
Copper(II)-Catalyzed Tandem Synthesis of Substituted 3-Methyleneisoindolin-1-ones
- Pages: 1022-1026
- First Published: 05 July 2013
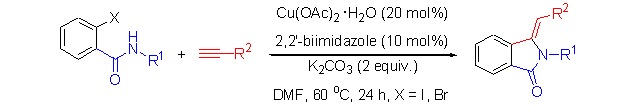
An efficient strategy for the synthesis of a variety of 3-methyleneisoindolin-1-ones has been developed. The reaction proceeded from coupling of 2-iodobenzamides (or 2-bromobenzamides) and terminal alkynes via Cu(OAc)2·H2O/2,2′-biimidazole catalyzed in DMF at 60°C and subsequent additive cyclization produced substituted 3-methyleneisoindolin-1-ones in good to excellent yields.
Synthesis of 5,6-Diarylpyridin-2(1H)-ones from Isoflavones
- Pages: 1027-1032
- First Published: 05 July 2013
Application of Functionalized N,S-Ketene Acetals–Microwave-Assisted Three-Component Domino Reaction for Rapid Direct Access to Imidazo[1,2-a]pyridines
- Pages: 1033-1038
- First Published: 17 June 2013
![Application of Functionalized N,S-Ketene Acetals–Microwave-Assisted Three-Component Domino Reaction for Rapid Direct Access to Imidazo[1,2-a]pyridines](/cms/asset/742c2f93-9e1f-4dcd-8d17-03e9c196eb63/mcontent.jpg)
Imidazo[1,2-a]pyridines have been successfully synthesized in moderate to good yields via a tandem three-component reaction of ethyl 2-(3-oxo-3-arylpropanethioamido)acetates with aromatic aldehydes and malononitrile under microwave irradiation using DABCO as the catalyst. The advantages of this method including high chemo- and regioselectivity make this new strategy highly attractive.
A Highly Diastereoselective Three-component Domino Reaction in Water Yielding Poly-substituted 4,5-Dihydropyrroles
- Pages: 1039-1044
- First Published: 17 June 2013
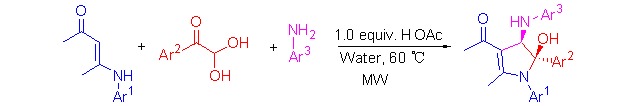
An efficient methodology for the highly diastereoselective synthesis of poly-substituted 4,5-dihydropyrrole derivatives from readily available common reactants in water has been developed. During domino processes, the formation of pyrrole skeleton and its C2-hydroxylation and C3-arylamination were readily achieved via metal-free [3+2] heterocyclization in a one-pot operation.
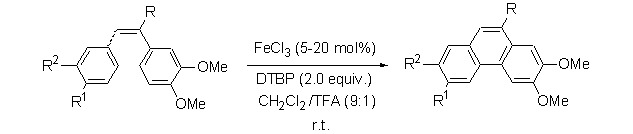
Iron(III) Chloride Catalyzed Oxidative Coupling Reaction of 1,2-Diarylethylene Derivatives
- Pages: 1045-1053
- First Published: 05 July 2013
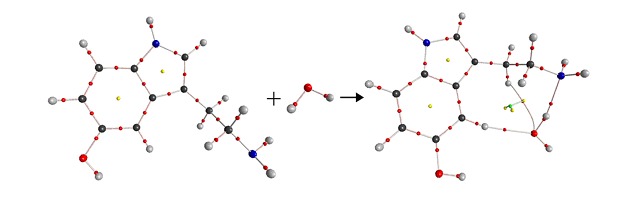
Efficient Synthesis of Spiro[furan-3,3′-indoline] Derivatives via Reactions of Pyridinium Salts with Isatinylidene Acetoacetates
- Pages: 1054-1058
- First Published: 19 July 2013
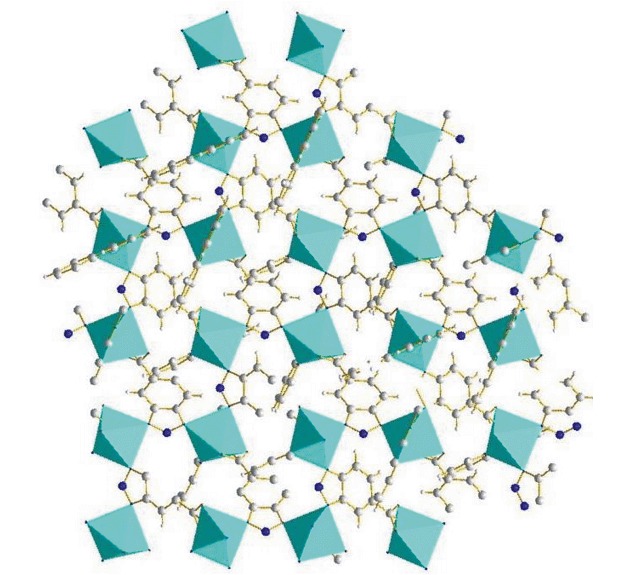
Syntheses, Structures and Properties of Four New Transitional Metal Complexes Based on Benzotriazole-5-carboxylic Acid
- Pages: 1059-1064
- First Published: 05 July 2013
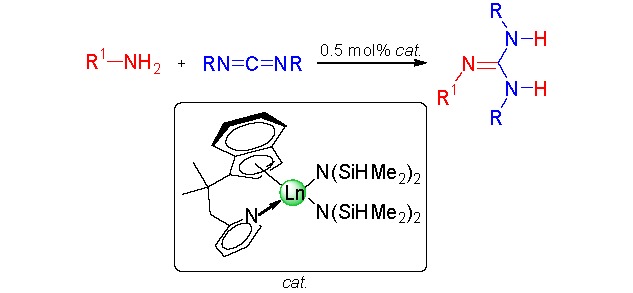
Synthesis of Guanidines from Amines and Carbodiimides Catalyzed by Mono-Indenyl-Ligated Rare Earth Metal Bis(silylamide) Complexes
- Pages: 1065-1071
- First Published: 17 June 2013
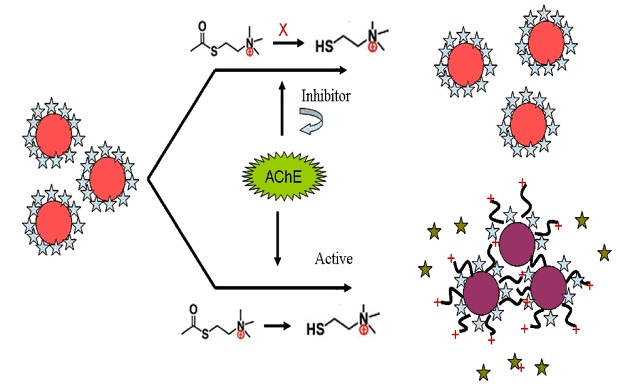
Highly Sensitive Fluorometric Assay Method for Acetylcholinesterase Inhibitor Based on Nile Red-Adsorbed Gold Nanoparticles
- Pages: 1072-1078
- First Published: 17 June 2013
Theoretical Study on the Hydrogen Bonding Interactions in Complexes of 5-Hydroxytryptamine with Water
- Pages: 1079-1086
- First Published: 29 May 2013
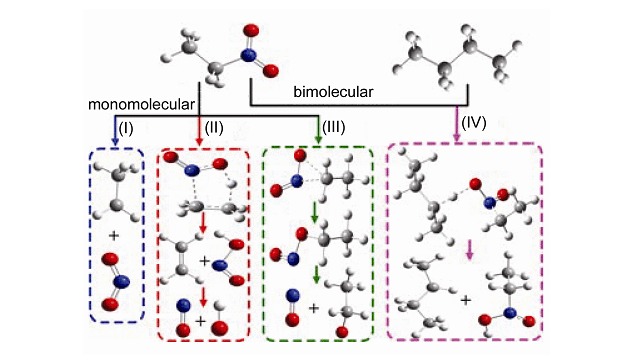
Understanding the Initial Decomposition Pathways of the n-Alkane/Nitroalkane Binary Mixture
- Pages: 1087-1094
- First Published: 05 July 2013
A Long-Wavelength Fluorescent Probe for Saccharides Based on Boronic-Acid Receptor
- Pages: 1095-1101
- First Published: 17 June 2013

A fluorescent sensor CSP for saccharides was designed and synthesized based on electron transfer mechanism. The conjugation was extended by incorporating an α,β-unsaturated ketone into the coumarin fluorophore. The sensor has good water-solubility, large Stocks shift (≈150 nm) and long emission wavelength (616 nm), which ensure CSP a potential sensor for sugars in biological systems.
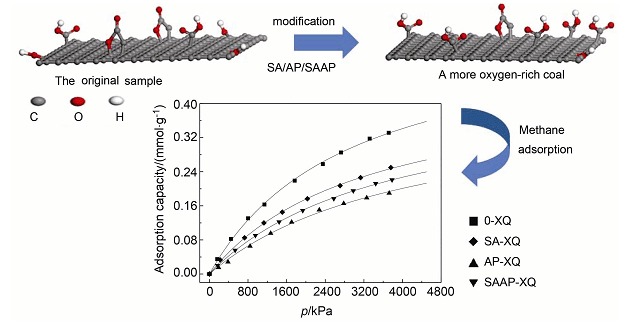
Surface Modification of Bituminous Coal and Its Effects on Methane Adsorption
- Pages: 1102-1108
- First Published: 05 July 2013





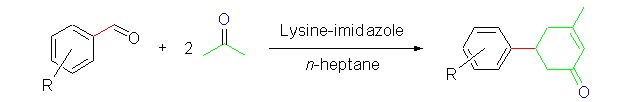
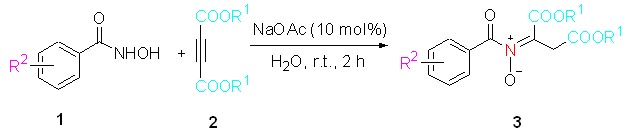
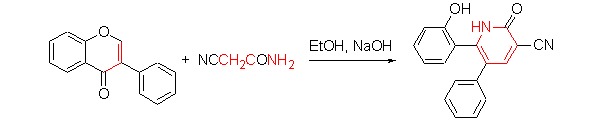
![Efficient Synthesis of Spiro[furan-3,3′-indoline] Derivatives via Reactions of Pyridinium Salts with Isatinylidene Acetoacetates](/cms/asset/50265a26-70ac-475f-81c7-305cf57c997d/mcontent.jpg)