Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture: Insight into the Structural Requirements of Protoporphyrinogen Oxidase Inhibitors: Molecular Docking and CoMFA of Diphenyl Ether, Isoxazole Phenyl, and Pyrazole Phenyl Ether (Chin. J. Chem. 9/2013)
- Page: 1113
- First Published: 18 September 2013

The cover picture shows that a multistep framework is developed with the ultimate goal of identifying novel herbicides. Protoporphyrinogen oxidase (PPO, EC 1.3.3.4) is one of the most significant targets for herbicide design. Using the crystal structure of tobacco mitochondrial PPO (mtPPO) as template, inhibitors were docked into the active site. The docking pose of each compound was subsequently used in a receptor-based alignment, leading to the development of a significant CoMFA model with r2 value of 0.98 and q2 (cross validation r2) value of 0.63. This novel multistep framework gives insight into the structural characteristics for the binding of inhibitors. In addition, the simplicity of the proposed approach may be particularly applicable in virtual screening procedures. More details are discussed in the article by Yang et al. on page 1153–1158.
Editorial
Special Issue of "Medicinal Chemistry"
- Page: 1115
- First Published: 18 September 2013
Contents
Contents: Chin. J. Chem. 9/2013
- Pages: 1116-1121
- First Published: 18 September 2013
Review
Molecular Similarity: Methods and Performance
- Pages: 1123-1132
- First Published: 16 September 2013
Communications
Synthesis of 4-(2-Phenylhydrazono)-1-(4-phenylthiazol-2-yl)-1H-pyrazol-5(4H)-one Compounds and Characterization of Their Affinities to Anti-apoptotic Bcl-2 Family Proteins
- Pages: 1133-1138
- First Published: 26 July 2013
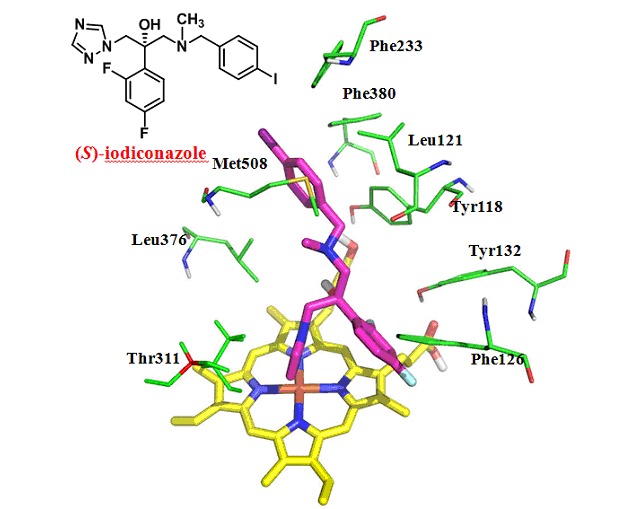
Asymmetric Synthesis, Antifungal Activity and Molecular Modeling of Iodiconazole Isomers
- Pages: 1139-1143
- First Published: 26 July 2013
Full Papers
Design and Synthesis of Tri-substituted Chiral Pyrrolidin-2-one Derivatives as CCR4 Antagonists
- Pages: 1144-1152
- First Published: 05 July 2013
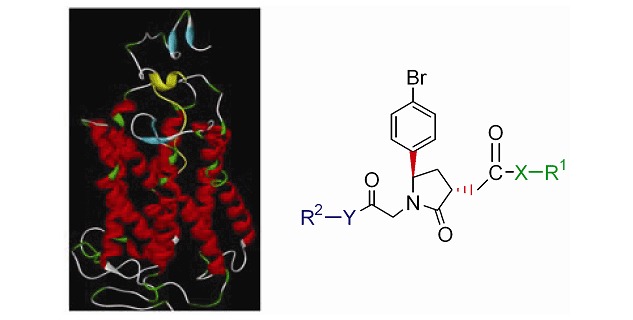
The structure of CCR4 based on bovine rhodopsin was built through homology modeling. And six tri-substituted chiral pyrrolidin-2-one derivatives have been designed and synthesized as CCR4 antagonists. Several of these compounds show high inhibitory effect in cell assays and higher affinity for the N-terminal of CCR4. Among the compounds, 1c exhibited excellent activity.
Insight into the Structural Requirements of Protoporphyrinogen Oxidase Inhibitors: Molecular Docking and CoMFA of Diphenyl Ether, Isoxazole Phenyl, and Pyrazole Phenyl Ether
- Pages: 1153-1158
- First Published: 16 September 2013
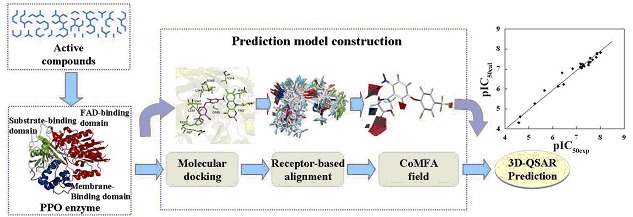
Protoporphyrinogen oxidase (PPO, EC 1.3.3.4) is one of the most significant action targets for a large and chemically diverse family of inhibitors that may be used as herbicide, bactericide, fungicide, or photosensitizing activator to treat cancer through photodynamic therapy (PDT). Molecular docking and 3D-QSAR CoMFA were combined in a multistep framework with the ultimate goal of identifying important factor contributing to the activity of PPO inhibitors. This novel multistep framework gives insight into the structural characteristics that affect the binding and the inhibitory activity of these analogues on PPO with different kinds of structural framework, and it can be extended to other classes of PPO inhibitors. In addition, the simplicity of the proposed approach may be particularly applicable in virtual screening procedures.
Synthesis and Preliminary Evaluation of 1-[18F]Fluoro-1-deoxy-2,5-anhydro-D-mannitol as a PET Radiotracer for Breast Cancer Imaging
- Pages: 1159-1163
- First Published: 16 September 2013
Design, Synthesis, and Biological Evaluation of Novel Dual Inhibitors of Secretory Phospholipase A2 and Sphingomyelin Synthase
- Pages: 1164-1170
- First Published: 03 April 2013
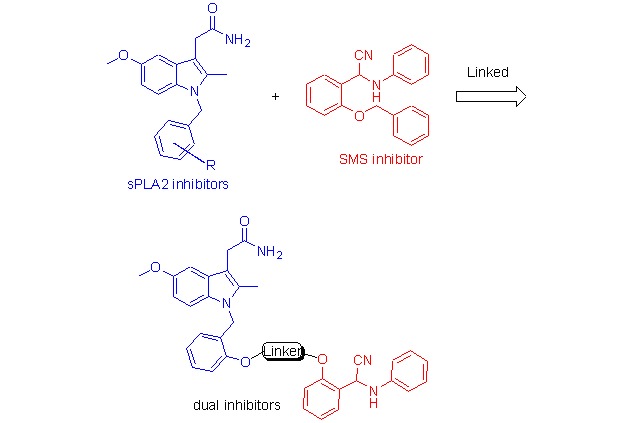
The secretory phospholipase A2 (sPLA2) and sphingomyelin (SMS) are the key enzymes to atherosclerosis. In this paper, we combined the single sPLA2 and SMS inhibitors with a carbon chain to get a series of dual inhibitors. The biological evaluation showed these compounds had the moderate inhibition against both enzymes.
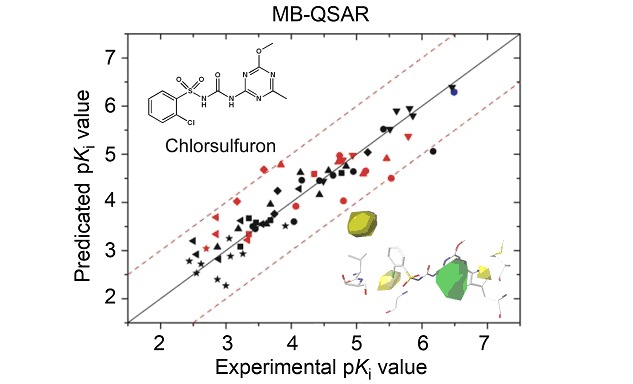
Molecular Drug Resistance Prediction for Acetohydroxyacid Synthase Mutants Against Chlorsulfuron Using MB-QSAR
- Pages: 1171-1180
- First Published: 16 September 2013
Design, Synthesis and Preliminary Biological Evaluation of Purine-2,6-diamine Derivatives as Cyclin-dependent Kinase (CDK) Inhibitors
- Pages: 1181-1191
- First Published: 16 September 2013
Triazole and Benzotriazole Derivatives as Novel Inhibitors for p90 Ribosomal S6 Protein Kinase 2: Synthesis, Molecular Docking and SAR Analysis
- Pages: 1192-1198
- First Published: 19 July 2013
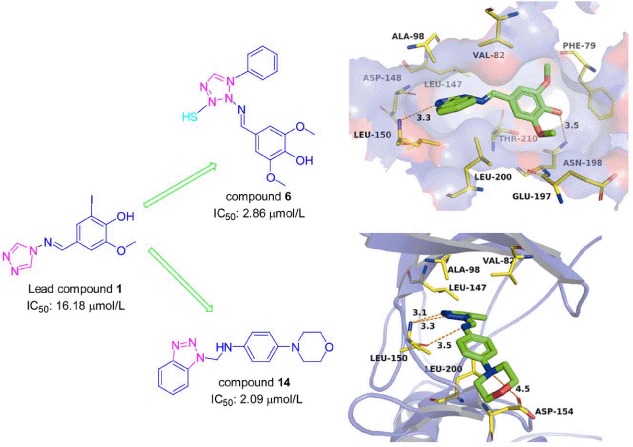
In the present study, a series of triazole and benzotriazole derivatives as novel p90 ribosomal S6 protein kinase 2 (RSK2) inhibitors were designed and synthesized. The in vitro activities of the 14 compounds against RSK2 were evaluated, among which, compounds 5, 6, 11, 12, 13 and 14 exhibited enzyme IC50 values of 8.91, 2.86, 3.19, 3.05, 4.49 and 2.09 µmol/L respectively. The proposed binding modes were simulated using molecular docking method, and the docking results coupled with the structure-activity relationship (SAR) analysis indicated that all our active compounds bound to the RSK2 ATP binding site at NTKD, and the electron-donating groups on the 4-position of phenyl were the key point for the inhibitory activity.
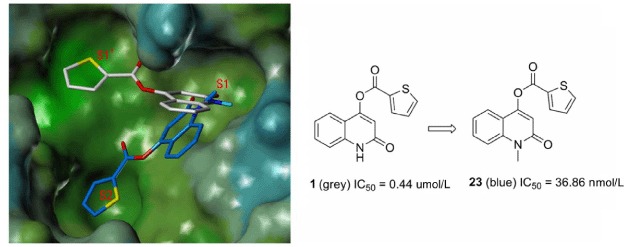
Synthesis and Biological Evaluation of Quinolinone Compounds as SARS CoV 3CLpro Inhibitors
- Pages: 1199-1206
- First Published: 19 July 2013
Synthesis and Anti-HIV Activity of a Series of 6-Modified 2′,3′-Dideoxyguanosine and 2′,3′-Didehydro-2′,3′-dideoxyguanosine Analogs
- Pages: 1207-1218
- First Published: 16 September 2013
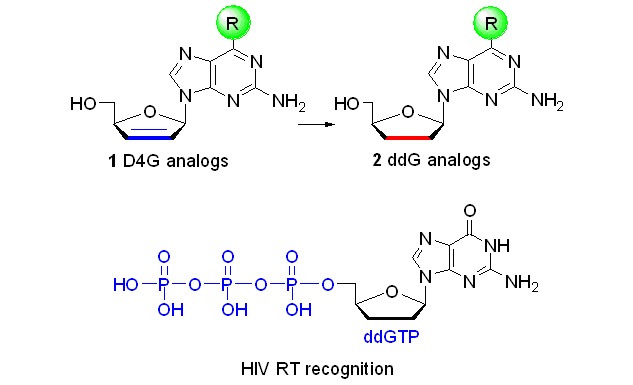
A series of 6-modified 2′,3′-dideoxyguanosine and 2′,3′-didehydro-2′,3′-dideoxyguanosine analogs were synthesized. Anti-HIV activity was investigated in cell-based assay. ddGTP was synthesized as well as D4TTP by a novel "one-pot" method, and the incorporation efficiencies recognized by DNA polymerase and HIV reverse transcriptase (HIV RT) were evaluated.
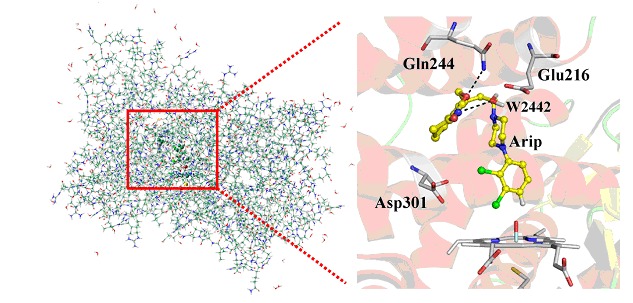
Catalytic Mechanism of Cytochrome P450 2D6 for 4-Hydroxylation of Aripiprazole: A QM/MM Study
- Pages: 1219-1227
- First Published: 16 September 2013
Synthesis, Biological Activity Evaluation and Molecular Modeling Study on the New Isoconessimine Derivatives as Acetylcholinesterase Inhibitors
- Pages: 1228-1233
- First Published: 16 September 2013
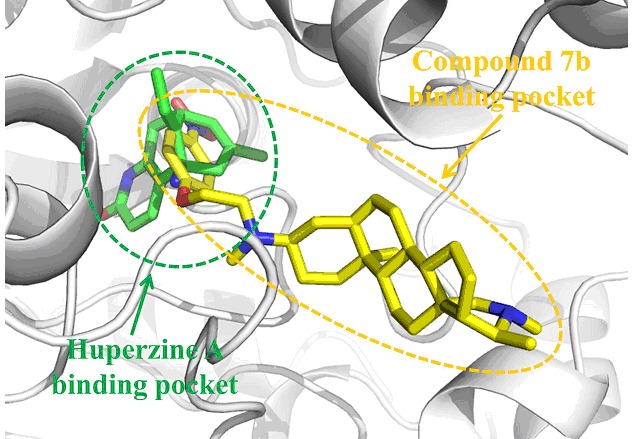
A series of 3-N-aryloxyethyl substitutional isoconessimine derivatives were synthesized and evaluated as acetylcholinesterase (AChE) inhibitors. All of the synthesized derivatives exhibited potential anti-acetylcholinesterase activities with IC50 values at micromolar to sub-micromolar range. 7b showed the most potent inhibitory activity with an IC50 of 110 nmol/L. The molecular docking results showed that 7b can be well docked into the active site of acetylcholinesterase.





![Synthesis and Preliminary Evaluation of 1-[18F]Fluoro-1-deoxy-2,5-anhydro-D-mannitol as a PET Radiotracer for Breast Cancer Imaging](/cms/asset/af17cfa7-0468-4b35-bdf8-0c33c9b1cd91/mcontent.jpg)