Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture: Palladium-Catalyzed Cross-Coupling of Fluorinated Vinyl Chlorides with Terminal Alkynes: A General Protocol to Fluorinated Enynes (Chin. J. Chem. 7/2013)
- Page: 865
- First Published: 22 July 2013

The cover picture shows an efficient and cost-effective method for the preparation of fluorinated conjugated enynes. The method was compatible with a variety of functional groups such as chloride, amine, cyano and ester group. The method could also be extended to the coupling of (R,Z)-3-(2-chloro-1,2-difluorovinyl)-4-alkyloxazolidin-2-one (3) with moderate yields. More details are discussed in the article by Shen et al. on page 901–907.
Editorial
Special Issue of "Organofluorine Chemistry"
- Page: 867
- First Published: 22 July 2013
Contents
Communication
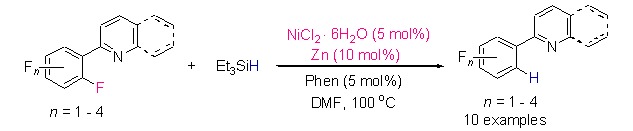
Nickel-Catalyzed ortho-Selective Hydrodefluorination of N-Containing Heterocycle-Polyfluoroarenes
- Pages: 873-877
- First Published: 05 July 2013
Full Papers
Highly Stereoselective and One-Pot Synthesis of Tetra-substituted Monofluoroalkenes with Aldehydes and Fluorobis(phenylsulfonyl)methane
- Pages: 878-884
- First Published: 05 July 2013
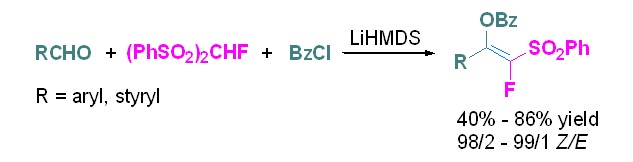
Highly stereoselective and one-pot synthesis of tetrasubstituted monofluoroalkenes with fluorobis(phenylsulfonyl)methane (FBSM) as the fluoroolefination reagent has been developed. This protocol is amenable to the transformation of non-enolizable aldehydes with less sterically demanding substituents. For enolizable aldehydes and sterically hindered aromatic aldehydes, the reaction furnishes carbonyl addition products.
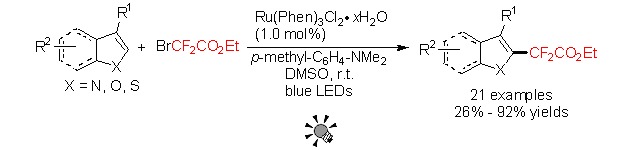
Direct Introduction of Ethoxycarbonyldifluoromethyl-Group to Heteroarenes with Ethyl Bromodifluoroacetate via Visible-Light Photocatalysis
- Pages: 885-891
- First Published: 19 July 2013
A General and Highly Selective Method for the Asymmetric Synthesis of Trifluoromethyl-Substituted α- and β-Aminophosphonates
- Pages: 892-900
- First Published: 19 July 2013
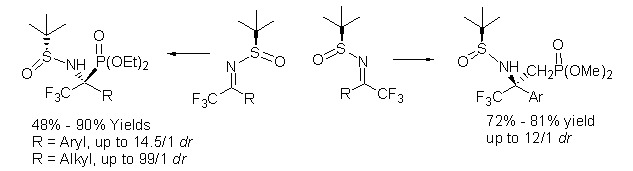
A highly selective method for the asymmetric formation of trifluoromethyl-substituted α- and β-aminophosphonates was described. The reaction proceeded with high yields and good to excellent diastereoselectivity. The product can be easily converted into corresponding trifluoromethyl-substituted aminophosphoric acids.
Palladium-Catalyzed Cross-Coupling of Fluorinated Vinyl Chlorides with Terminal Alkynes: A General Protocol to Fluorinated Enynes
- Pages: 901-907
- First Published: 19 July 2013
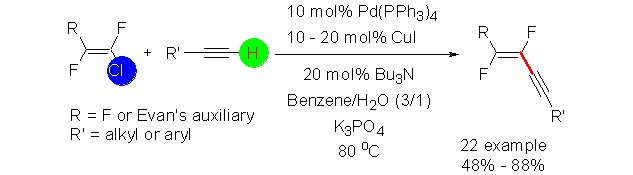
An efficient and cost-effective method for the preparation of fluorinated conjugated enynes was described. The method was compatible with a variety of functional groups such as chloride, amine, cyano and ester group. The method could also be extended to the coupling of (R,Z)-3-(2-chloro-1,2-difluorovinyl)-4-alkyloxazolidin-2-one (3) with moderate yields.
Palladium-Catalyzed Intramolecular Fluorooxylation of Styrenes
- Pages: 908-914
- First Published: 05 July 2013
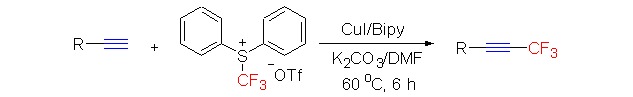
Copper-Mediated Trifluoromethylation of Terminal Alkynes by S-(Trifluoromethyl)diarylsulfonium Salt
- Pages: 915-920
- First Published: 19 July 2013
Nucleophilic Trifluoromethylthiolation of Allylic Bromides: A Facile Preparation of Allylic Trifluoromethyl Thioethers
- Pages: 921-926
- First Published: 05 July 2013
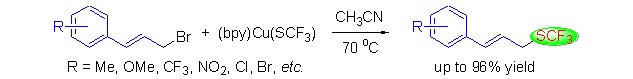
A facile preparation of allylic trifluoromethyl thioethers was achieved by the reaction of (bpy)Cu(SCF3) with allylic bromides. This protocol afforded the desired trifluoromethylthiolation products in good to excellent yields with high stereo- and regioselectivity. Important functional groups such as alkyl, alkoxy, trifluoromethyl, nitro, halides and geranyl are well tolerated.
Cobalt-catalyzed Selective CF Bond Activation and Alkylation of Polyfluoroaryl Imines
- Pages: 927-932
- First Published: 19 July 2013
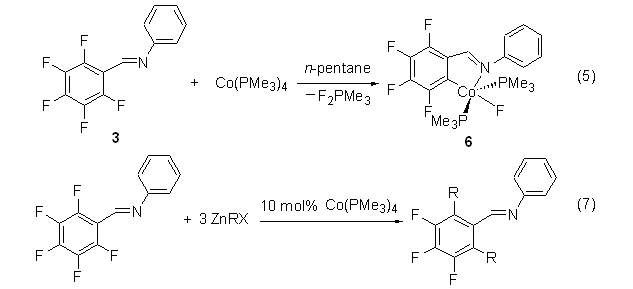
CF bond activation of polyfluoroaryl imines is explored at cobalt(0) center with an imine-N atom as an anchoring group. It was also found that dialkylation of N-(perfluorobenzylidene)benzenamine with organozinc reagents could be catalyzed by Co(PMe3)4 via C,C-coupling reaction under mild conditions.
Fluorocarbon and Hydrocarbon Benzodioxocycloalkane (C8–C10) End Groups: Effects on Mesomorphism
- Pages: 933-938
- First Published: 19 July 2013
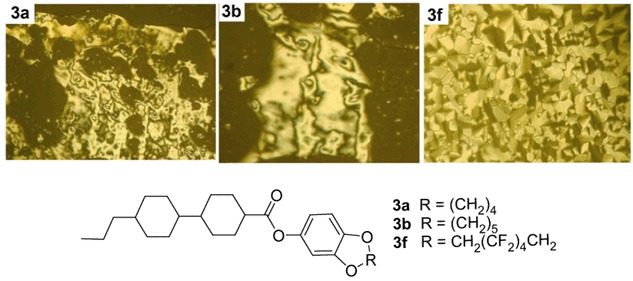
A new class of benzodioxocycloalkane-based (C8–C10) liquid crystals has been prepared. The impact of ring (C8–C10) as end group was investigated. The 8–9 membered ring derivatives, 3a–3b, exhibited the nematic phases (N). The mesomorphic behaviors were weakened with increasing the size of the ring. For the ?uorinated medium ring (C8–C10) 3d–3f, it was found only the fluorinated ten membered ring 3f showed the LC phases behaviours. Modification of the ring size with different length alkyl groups and variation of the alkyl to polyfluoroalkyl markedly influenced the properties of these compounds.
Radical Addition of Perfluoroalkyl Iodides to Alkenes and Alkynes Initiated by Sodium Dithionite in an Aqueous Solution in the Presence of a Novel Fluorosurfactant
- Pages: 939-944
- First Published: 19 July 2013
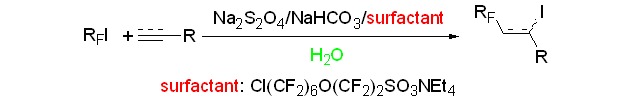
A new surfactant Cl(CF2)6O(CF2)2SO3N(C2H5)4 was prepared and its properties were measured. The fluorinated surfactant was applied to the fluoroalkylation of perfluoroalkyl iodides with olefins and alkynes. The reactions gave the corresponding adducts in water and avoided the use of organic solvent. The aqueous solution with the surfactant proved to be an effective medium for a radical process.
Copper(I)-Catalyzed Trifluoromethylation of Phthalic Anhydride Derivatives with (Trifluoromethyl)trimethylsilane
- Pages: 945-949
- First Published: 05 July 2013
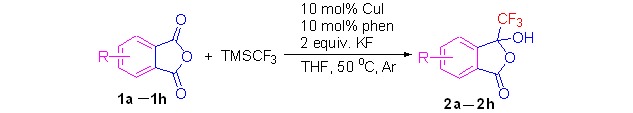
An efficient copper-mediated trifluoromethylation of substituted phthalic anhydrides using (trifluoromethyl)trimethylsilane (Me3SiCF3) as a nucleophilic trifluoromethylating reagent in the presence of 1,10-phenanthroline and KF, followed by quenching the reaction mixture with water has been developed. A possible mechanism was suggested.
Regioselective Synthesis of 2,6-Dimethyl-3,5-bis[(3-aryl-5-trifluoromethyl)-isoxazol-4-carbonyl]-pyridine Derivatives
- Pages: 950-954
- First Published: 05 July 2013





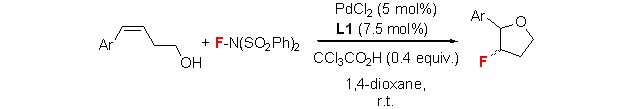
![Regioselective Synthesis of 2,6-Dimethyl-3,5-bis[(3-aryl-5-trifluoromethyl)-isoxazol-4-carbonyl]-pyridine Derivatives](/cms/asset/c4d4b9cc-7ae4-4dd6-8413-4ef30060bfa1/mcontent.jpg)




