Journal list menu
Press Release
Angewandte Chemie International Edition
doi.org/10.1002/anie.202103960
Nr. 20/2021
June 21, 2021
Molecular Assistance
Molecule layer aids chemoselective hydrogenation on solid palladium catalysts
© Wiley-VCH, re-use with credit to 'Angewandte Chemie' and a link to the original article.
Chemical reactions don’t always go to plan. Unwanted by-products lead to extra costs and waste resources. Selective catalysts can help, but chemists have to test out large numbers before they find the right fit. Researchers have now investigated, on an atomic level, how to obtain a palladium catalyst for the selective hydrogenation of acrolein. The key appears to be a dense, convertible layer of ligand molecules, report the authors in the journal Angewandte Chemie.
Contact: Swetlana Schauermann, Christian-Albrechts-Universität zu Kiel (Germany)
Registered journalists may download the original article here:
Understanding Ligand-Directed Heterogeneous Catalysis: When the Dynamically Changing Nature of the Ligand Layer Controls the Hydrogenation Selectivity
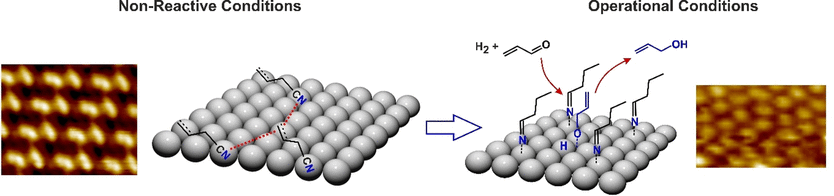
The acrolein molecule has two positions where it can be hydrogenated. When reacted with hydrogen, either the alcohol, propenol, or the aldehyde, propanal, is formed. Palladium catalysts can be used to steer the reaction toward propenol, but scientists have observed that this only works if the surface of the metal has already been coated with the reaction partner or a similar hydrocarbon as a ligand precursor. Swetlana Schauermann and her team at the University of Kiel, Germany, have now investigated why this is the case and what actually happens in this reaction.
For the team’s experiments, they first coated pure palladium metal with allyl cyanide, the ligand precursor for the reaction. To visualize this coating in detail, the researchers analyzed the palladium surface using scanning tunneling microscopy. The results showed a “flat” coating of the allyl cyanide where all three carbon atoms of the allyl, as well as the cyanide functional group, lie flat on the metal atoms. No protrusions from the surface were noted.
This flat ligand layer changed when the metal was exposed to the reaction conditions and a stream of hydrogen was passed over the surface of the metal. Scanning tunneling microscopy revealed a dense coating, but with considerably shorter distances between the molecules. The researchers used the type of changes taking place, and spectroscopic analyses, to work out exactly what was going on. The hydrogen had hydrogenated the allyl cyanide molecule and converted it into a saturated hydrocarbon with an imine functional group.
The imine was no longer lying flat on the surface though: it was standing up. This happened because the end of the molecule with the saturated hydrocarbon residue had lost contact with the palladium atoms, while the imine function remained bonded to the metal. The flat surface of the catalyst had transformed into a forest of upright molecular trees.
This new coating activated the catalyst, enabling precise positional docking of the acrolein and activation of the oxygen function ready for hydrogenation. “On this active layer, acrolein nearly instantaneously forms the desired propenoxy reaction intermediate followed by evolution of the target product propenol,” the authors observed.
The chemoselectivity and activity of the palladium catalyst could be explained in detail. “This is the first experimental proof of the formation of an active ligand layer obtained by real space microscopy,” state the authors. The team hopes this new, deeper understanding could be used to find other functionalizations to improve the chemoselectivity of metal catalysts.
(3180 characters)
About the Author
Prof. Dr. Swetlana Schauermann is a Professor of Physical Chemistry at the University of Kiel, Germany. Her research activities are directed towards a better understanding of the foundations of heterogeneous catalysis in order to gain an atomic-scale knowledge of the elementary processes of chemical reactions and to create and modify solid-state catalysts so that only the desired reactions can take place.
Copy free of charge—we would appreciate a transcript of your article. The original articles that our press releases are based on can be found in our online pressroom at http://pressroom.angewandte.org.