Journal list menu
Press Release
Angewandte Chemie International Edition ,
doi: 10.1002/anie.201603211
Nr. 21/2016
June 27, 2016
High-Speed Tunnel for Carbon Dioxide
Highly permeable mineral/polymer membrane for CO2 separation
Contact: Zhi Wang, Tianjin University (China)
Registered journalists may download the original article here:
A Highly Permeable Aligned Montmorillonite Mixed-Matrix Membrane for CO2 Separation
To keep greenhouse gas carbon dioxide from entering the environment, it must be stored, or even better used as a raw material. The first step in such processes is the efficient separation of CO2 from exhaust leaving power plants and factories. One current trend is to explore using membrane separation processes. In China, a new approach for making novel membranes based on multilayered materials and polymers has now been developed. As the scientists report in the journal Angewandte Chemie, the cavities between the mineral layers serve as selective transport channels for CO2.
© Wiley-VCH
The separation of CO2 plays a role in many technical processes aside from dealing with exhaust. In order to make natural gas or biogas usable for various technical applications, CO2, a destructive, acidic impurity, must be removed. In some equilibrium reactions, such as the production of synthesis gas, the removal of CO2 from the mixture can “drive” the desired reaction.
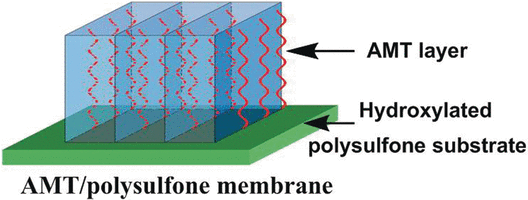
Membrane separation processes are gaining in popularity because they use less energy and are simpler than conventional separation methods. The key to their success lies in porous materials with highly selective permeability. Clays with a layered structure, such as montmorillonite, are intriguing candidates for the separation of CO2: The spaces between their layers form linear channels containing exchangeable ions. The choice of ion can be used to control the width of the channels. Furthermore, the walls of the channels contain hydroxy groups (OH), which have a high affinity for CO2 molecules. The construction of selective, high-speed transport channels for CO2 depends on the mineral having the right alignment.
A team headed by Zhi Wang and Michael D. Guiver has now achieved this. They succeeded by aligning and embedding the aluminum- and magnesium-containing layered silicate called montmorillonite in a polymer matrix on a substrate.
The researchers from Tianjin University and the Collaborative Innovation Center of Chemical Science and Engineering in Tianjin, China, first stuck polyvinylamineacid chains onto a porous polymer substrate. These polymer chains are aligned perpendicular to the substrate. Between the chains, the researchers introduced nanoparticles of montmorillonite in which they had previously replaced the usual calcium ions with sodium ions. A special coupling reagent was used to bind the mineral layers with the polymer chains into a mixed matrix. This method maintains the layers, and thus their channels, parallel to the polymer chains. The result is an ultrathin membrane with straight channels that can be used for transport.
The OH groups in the channel walls interact with the acidic CO2, facilitating its transport. The permeability of the membrane for CO2 is thus significantly higher than for nitrogen, methane, or hydrogen, which allows the CO2 to be separated from mixtures.
(2967 characters)
About the Author
Dr. Zhi Wang is a Professor at the School of Chemical Engineering and Technology, Tianjin University, and has been working on the development of novel separation membranes and membrane processes for over 20 years, especially for CO2 separations. He is the Director of the Tianjin Key Laboratory of Membrane Science and Desalination Technology.






































