Journal list menu
Press Release
Angewandte Chemie International Edition ,
doi: 10.1002/anie.201602745
Nr. 17/2016
June 17, 2016
Loadable Nanocapsules
Carbon nanobubbles as containers with unusual loading and release properties
Contact: Wendelin Stark, ETH Zürich (Switzerland)
Registered journalists may download the original article here:
Hollow Carbon Nanobubbles: Synthesis, Chemical Functionalization and Container-Type Behavior in Water
Nanocapsules are used, for example, to protect sensitive products in cosmetics and foods, or to deploy pharmaceuticals in a targeted way by releasing them into the body only under specific conditions. In the journal Angewandte Chemie, Swiss scientists have now introduced a new variety of nanocapsule: hollow carbon bubbles made from three to four graphitic layers of carbon atoms. These bubbles are very soluble in water and can spontaneously take up hydrophobic molecules, releasing them only when their concentration in the surroundings gets very low. These are good properties for a drug transport system that releases its cargo over a longer time span.
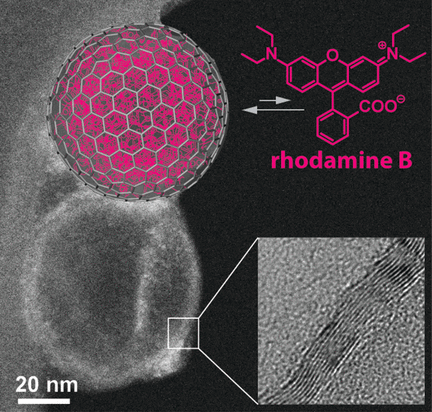
© Wiley-VCH
Nanocapsules based on pure carbon are particularly interesting because they are inert and biocompatible. The most famous hollow carbon spheres are the fullerenes. Their basic structural element is a hexagonal honeycomb that resembles a single layer of graphite. However, fullerenes are much too small to serve as capsules. Other, larger, hollow carbon spheres often have large holes, have too low a proportion of graphitic structures, or can’t be loaded with organic molecules.
Scientists working with Wendelin J. Stark at the ETH Zurich have now developed a new type of graphitic carbon nanocapsule with a hydrophobic interior and a hydrophilic exterior. This is ideal for drugs that are not soluble in water but must be transported through the aqueous environment in our bodies.
The synthesis begins with carbon-coated cobalt nanoparticles, onto which the researchers allow negatively charged polymer chains to grow. The presence of the metal core, means that this happens exclusively on the surface of the bubble. The chains ensure high solubility in water and prevent the capsules from clumping together. At the end of the process, the metal cores are washed away with acid.
Loading the capsules with rhodamine B led to an interesting effect: the hydrophobic fluorescence dye spontaneously concentrated within the bubbles—against the concentration gradient. Apparently, these bubbles behave like oil droplets in an aqueous phase. Substances that have low solubility in water preferentially drift into the oil phase.
The dye is released only when the external concentration is drastically reduced. Loading and unloading demonstrate an unexpected hysteresis. The researchers suggest that the rhodamine B accumulated within the bubbles forms something like a drop, analogous to an oil-in-water emulsion. In this form, the dye is in a relatively stable state. Release thus only happens at much lower concentrations in the surrounding solution than would be expected from the loading process.
These loadable nanocapsules are mechanically stable and form stable emulsions with a nearly unlimited lifetime. They are an interesting new approach for the production of novel nanotransportation systems.
(2945 characters)
About the Author
Dr. Wendelin Stark is Professor for Functional Materials Engineering at ETH Zurich and has been working with small particles for over 15 years. He is a member of the United Nations' Scientific Advisory Panel for the ongoing Global Environmental Outlook and has translated research findings into over 20 commercial products leading to 5 spin-off companies.






































