Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture: Palladium-Catalyzed Intermolecular Oxidative Coupling Reactions of (Z)-Enamines with Isocyanides through Selective β-C(sp2)-H and/or C=C Bond Cleavage (Chin. J. Chem. 8/2018)
- Page: 673
- First Published: 13 July 2018

The cover picture shows Two efficient intermolecular oxidative coupling reactions of (Z)-enamines with isocyanides via palladium catalysis have been developed. In these transformations, the β-C(sp2)-H and/or C = C bond were cut off selectively by using different anionic ligands, leading to controllable chemodivergent and stereoselective construction of a wide range of (E)β-carbamoylenamine derivatives with intramolecular hydrogen bonds. More details are discussed in the article by Jiang et al. on page 712–715.
Inside Cover
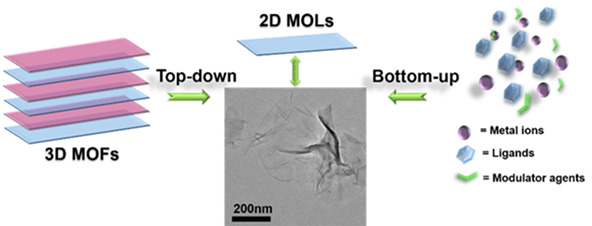
Inside Cover: Synthetic Strategies for Constructing Two-Dimensional Metal-Organic Layers (MOLs): A Tutorial Review (Chin. J. Chem. 8/2018)
- Page: 674
- First Published: 13 July 2018

The inside cover picture shows Metal-organic layer (MOL), the two-dimensional analog of metal-organic framework (MOF), is a new member of two-dimensional materials. This tutorial review summarizes current synthetic approaches for MOL preparation. More details are discussed in the article by Wang et al. on page 754–764.
Contents
Chemistry Authors Up Close
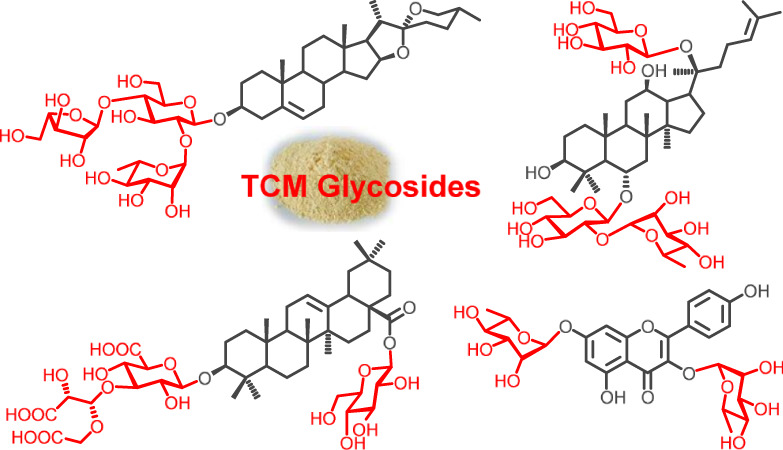
Synthesis of the Diverse Glycosides in Traditional Chinese Medicine
- Pages: 681-691
- First Published: 03 May 2018
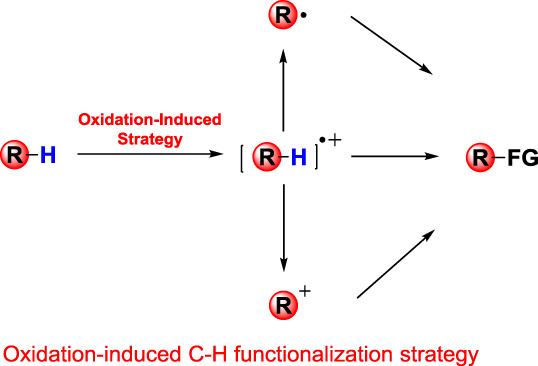
Oxidation-Induced C—H Functionalization: A Formal Way for C—H Activation
- Pages: 692-697
- First Published: 03 May 2018
Comprehensive Reports
Multiple Hydrogen Bonds Promoted ESIPT and AIE-active Chiral Salicylaldehyde Hydrazide
- Pages: 698-707
- First Published: 10 May 2018
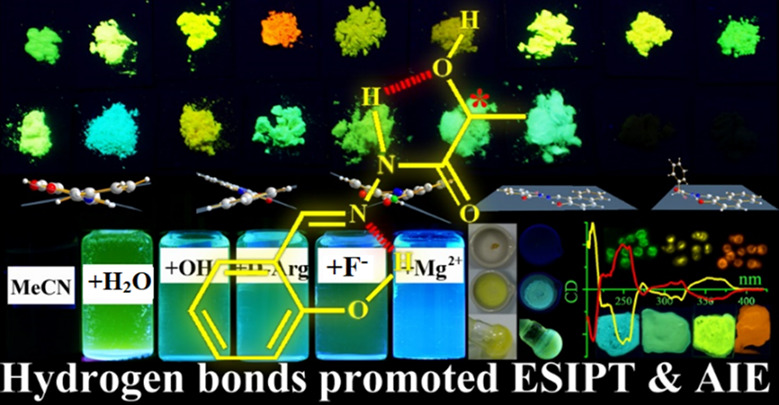
The simpler, the better! In the present work, we firstly reported a series of simple and soft salicylaldehyde hydrazide materials (41 samples) that exhibit multiple intramolecular and intermolecular hydrogen bonds promoted ESIPT and AIE properties with large Stokes shifts and can be used as multiple-stimuli-responsive smart materials for many applications in mechanochromism, universal probes, chiral recognition, and living cell imaging.
Palladium-Catalyzed Formal [4+2] Cycloaddition of Benzoic and Acrylic Acids with 1,3-Dienes via C—H Bond Activation: Efficient Access to 3,4-Dihydroisocoumarin and 5,6-Dihydrocoumalins
- Pages: 708-711
- First Published: 21 May 2018
![Palladium-Catalyzed Formal [4+2] Cycloaddition of Benzoic and Acrylic Acids with 1,3-Dienes via C—H Bond Activation: Efficient Access to 3,4-Dihydroisocoumarin and 5,6-Dihydrocoumalins](/cms/asset/7e0f9890-0538-46fb-9d26-0d97657e699c/cjoc201800149-toc-0001-m.jpg)
We report a palladium-catalyzed formal intermolecular [4+2] cycloaddition of benzoic and acrylic acids with 1,3-dienes including the stock chemicals 1,3-butadiene and isoprene leading to synthetically useful 3,4-dihydroisocoumarin and 5,6-dihydrocoumalins. Stepwise C—H bond cleavage and annulation are likely involved in the reaction pathway. The synthetic potential of the methodology was demonstrated by two short derivatizations and total synthesis of natural product Clausamine B.
Palladium-Catalyzed Intermolecular Oxidative Coupling Reactions of (Z)-Enamines with Isocyanides through Selective β-C(sp2)-H and/or C=C Bond Cleavage
- Pages: 712-715
- First Published: 10 May 2018
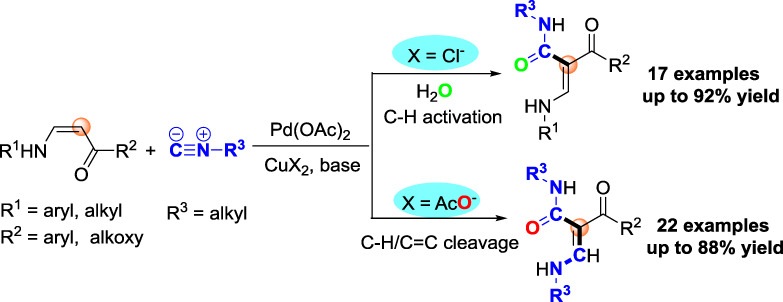
Herein, two efficient palladium-catalyzed intermolecular oxidative coupling reactions of (Z)-enamines with isocyanides via selective β-C(sp2)-H and/or C=C bond cleavage have been developed. A wide range of (E)-β-carbamoylenamine derivatives containing strong intramolecular hydrogen bonds were constructed in a chemodivergent manner.
Concise Reports
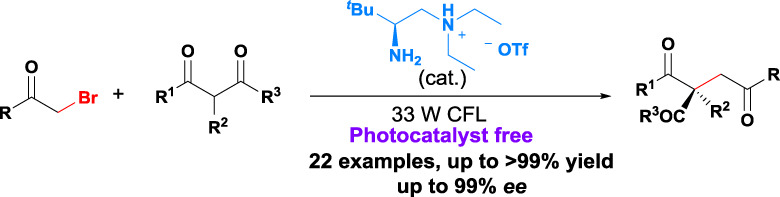
Asymmetric α-Alkylation of β-Ketocarbonyls via Direct Phenacyl Bromide Photolysis by Chiral Primary Amine
- Pages: 716-722
- First Published: 27 April 2018
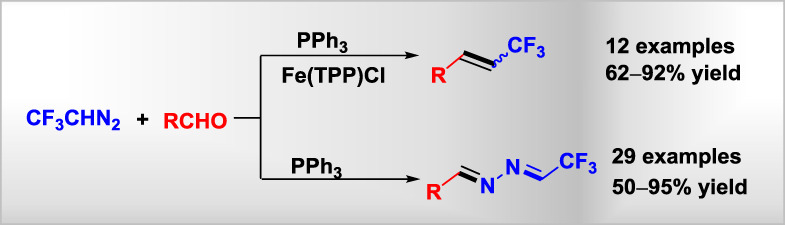
Phosphine-Relayed Aldehyde-Olefination and Aza-Wittig Reaction with 2,2,2-Trifluorodiazoethane
- Pages: 723-730
- First Published: 26 May 2018
Design, Synthesis and Biological Evaluation of Isothiazole Based 1,2,4-Trizaole Derivatives
- Pages: 731-736
- First Published: 15 May 2018
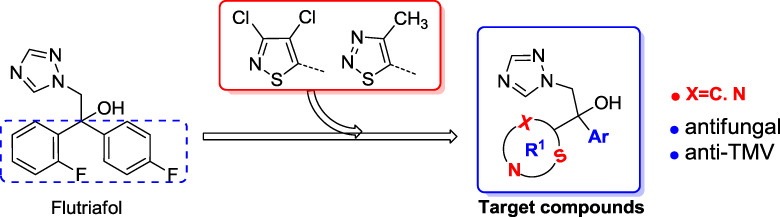
The novel 3,4-dichloroisothiazole based 1,2,4-trizaole derivatives were designed, synthesized and their in vitro antifungical activities and in vivo anti-TMV activity were evaluated. The bioassay results indicated that compound 6b, namely 1-(3,4-dichloroisothiazol-5-yl)-1-(4-fluorophenyl)-2- (1H-1,2,4-triazol-1-yl)ethanol exhibited both excellent fungicidal activity against B. cinerea, C. arachidicola and P. piricola with EC50 values of 6.98, 2.73 and 3.07 μg/mL, respectively, and more than 60% inactivation and induction anti-TMV activity at 100 μg/mL. Therefore, compound 6b could be a promising fungicidal and anti-TMV candidate worthy of further studies.
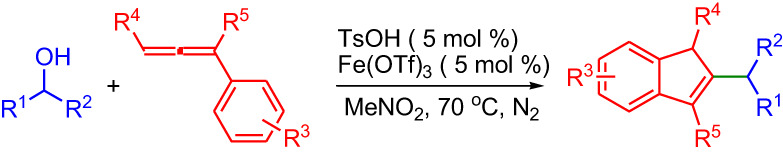
TfOH/Fe(OTf)3 Cocatalyzed Reaction of Arylallenes with Alcohols for Structurally Diverse Indene Derivatives
- Pages: 737-742
- First Published: 04 May 2018
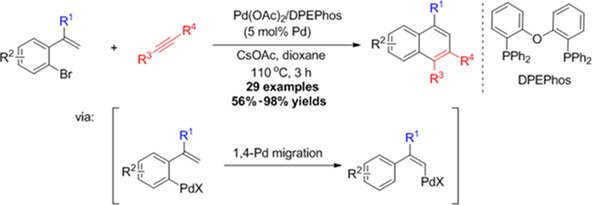
Synthesis of Substituted Naphthalenes by 1,4-Palladium Migration Involved Annulation with Internal Alkynes
- Pages: 743-748
- First Published: 03 June 2018
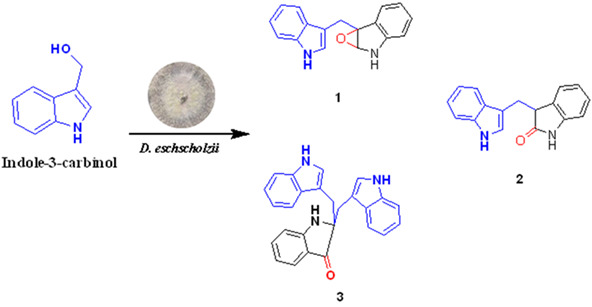
Bioactive Alkaloids from Indole-3-carbinol Exposed Culture of Daldiniaeschscholzii
- Pages: 749-753
- First Published: 26 May 2018
Recent Advances
Synthetic Strategies for Constructing Two-Dimensional Metal-Organic Layers (MOLs): A Tutorial Review
- Pages: 754-764
- First Published: 23 May 2018
Meeting Our New Members of Editorial Board of Rising Stars
Meeting Our New Members of Editorial Board of Rising Stars
- Pages: 765-774
- First Published: 13 July 2018
Back Cover
Back Cover: Bioactive Alkaloids from Indole-3-carbinol Exposed Culture of Daldiniaeschscholzii (Chin. J. Chem. 8/2018)
- Page: 780
- First Published: 13 July 2018
The back cover picture shows Two new and rare bioactive indoles named dalesindoloids A (1) and B (3), along with 3-(1H-indole-3ylmethyl)-2-oxindole (2), were characterized from the indole-3-carbinol (I3C)-exposed culture of Daldinia eschscholzii. Dalesindoloids A and B were cytotoxic against the leukemia HL-60 cell line with the IC50 values of 1.0 and 7.4 mol/L, respectively, with the former being inhibitory on Staphylococcus aureus (MIC: 9.1 μmol/L). The simultaneous characterization of the alkaloids from the I3C-exposed fungal culture highlighted that the 2,3-epoxyindoline motif can be transformed into both lactam and indolin-3-one moieties. This is the first-time description of the 2,3-epoxyindoline chemical versatility and Wagner-Meerwein rearrangement (WMR) reaction in the microbial culture. More details are discussed in the article by Tan et al. on page 749–753.