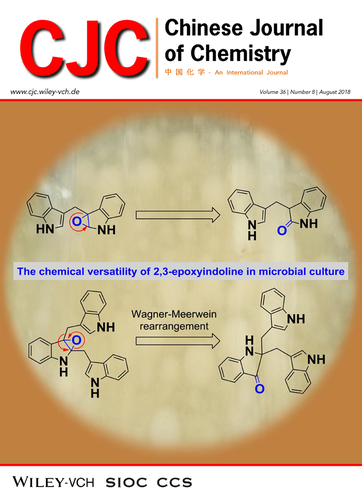
Back Cover: Bioactive Alkaloids from Indole-3-carbinol Exposed Culture of Daldiniaeschscholzii (Chin. J. Chem. 8/2018)
Abstract
The back cover picture shows Two new and rare bioactive indoles named dalesindoloids A (1) and B (3), along with 3-(1H-indole-3ylmethyl)-2-oxindole (2), were characterized from the indole-3-carbinol (I3C)-exposed culture of Daldinia eschscholzii. Dalesindoloids A and B were cytotoxic against the leukemia HL-60 cell line with the IC50 values of 1.0 and 7.4 mol/L, respectively, with the former being inhibitory on Staphylococcus aureus (MIC: 9.1 μmol/L). The simultaneous characterization of the alkaloids from the I3C-exposed fungal culture highlighted that the 2,3-epoxyindoline motif can be transformed into both lactam and indolin-3-one moieties. This is the first-time description of the 2,3-epoxyindoline chemical versatility and Wagner-Meerwein rearrangement (WMR) reaction in the microbial culture. More details are discussed in the article by Tan et al. on page 749–753.