Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture: BF3·Et2O Promoted Sulfuration of Steroidal Sapogenins (Chin. J. Chem. 6/2015)
- Page: 617
- First Published: 17 June 2015
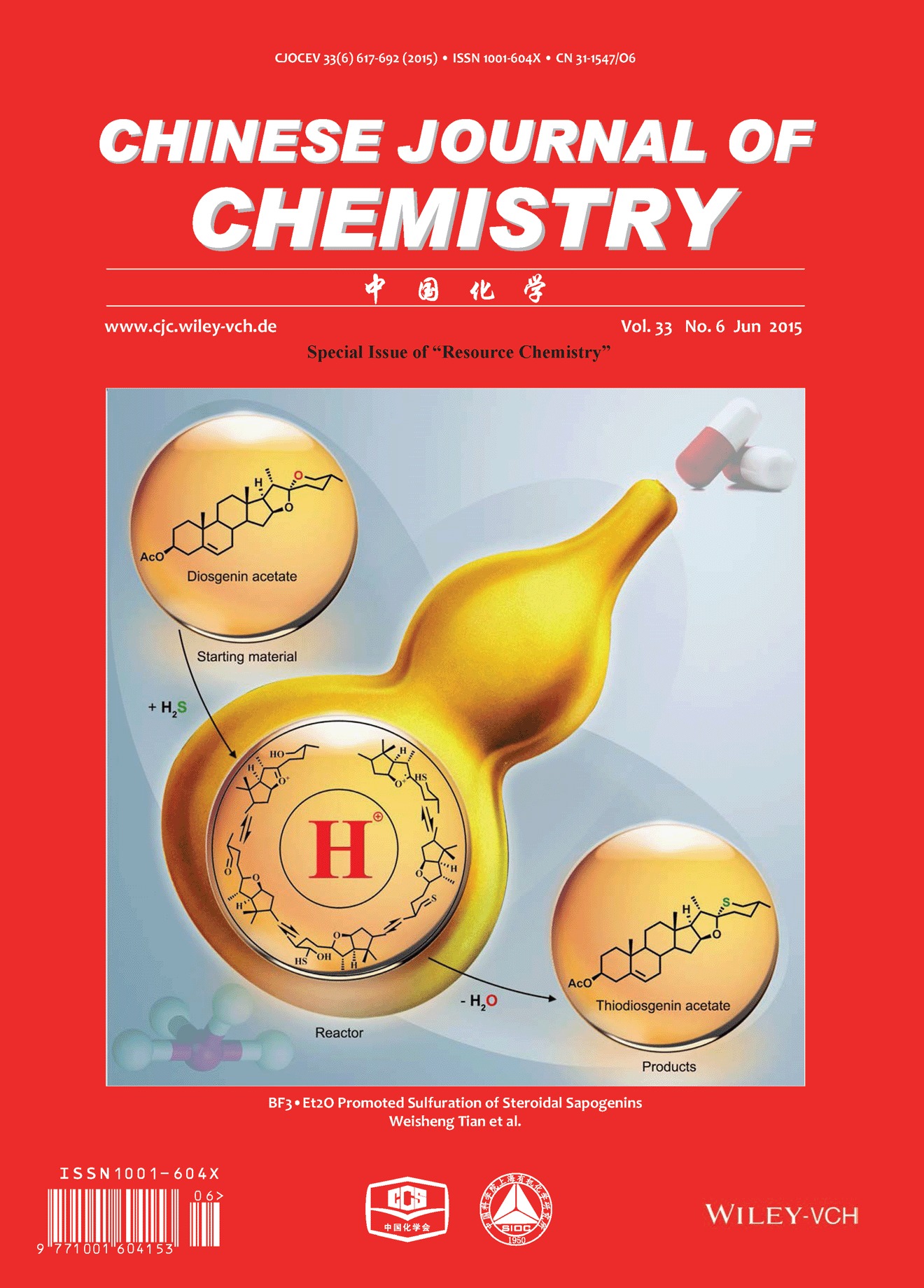
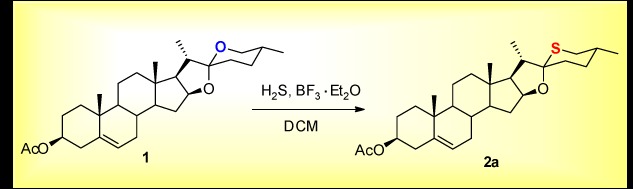
The cover picture shows a direct synthesis of 26-thio steroidal sapogenins from natural steroidal sapogenins, which serve as an important kind of resource compounds and basic starting materials in steroidal pharmaceutical industry. This method was based on a key and magic BF3·Et2O promoted C-26 sulfuration of steroidal sapogenins, which resulted in the direct replacement of the oxygen atom in F-ring of steroidal sapogenins by sulfur. The synthesis not only provides a concise method for 26-thio steroidal sapogenins, but also presents an ideal model of the utilization of steroidal sapogenins. More details are discussed in the article by Wang and Tian et al. on page 632–636.
Editorial
Contents
Communications
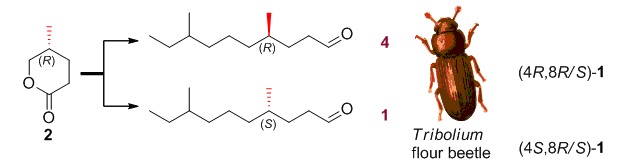
Synthesis of Tribolure, the Common Aggregation Pheromone of Four Tribolium Flour Beetles
- Pages: 627-631
- First Published: 10 June 2015
BF3·Et2O Promoted Sulfuration of Steroidal Sapogenins
- Pages: 632-636
- First Published: 02 June 2015
A Formal Synthesis of Betamethasone
- Pages: 637-642
- First Published: 02 June 2015
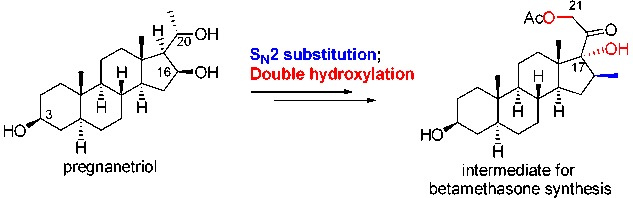
A formal synthesis of betamethasone from pregnane-3β,16β,20S-triol is described. Key transformations are a bromination-acetylation of triol, an SN2 reaction of the resulting C16α-bromide with dimethylcopperlithium to get the required C16β-methyl group, and a double hydroxylation to prepare the dihydroxyacetone side chain.
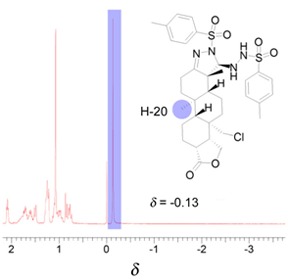
Methyl Group in Isocopalane Derivative Showed an Unusual Negative 1H NMR Chemical Shift
- Pages: 643-645
- First Published: 05 May 2015
Full Papers
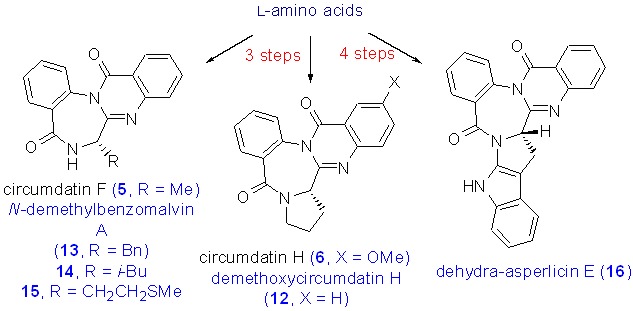
Low-Valent Titanium-Mediated Enantioselective Synthesis of Quinazolinone Alkaloids Circumdatins F, H, and Analogs
- Pages: 646-654
- First Published: 09 March 2015
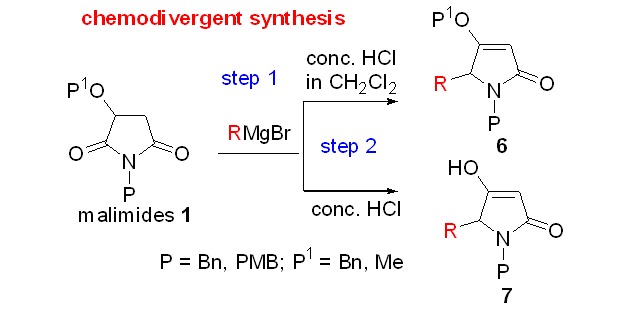
Towards Reaction Control: An Expeditious Access to Racemic 5-Substituted Tetramates and 5-Substituted Tetramic Acids from Malimides
- Pages: 655-662
- First Published: 12 December 2014
Synthesis of C1–C9 Domain of the Nominal Didemnaketal A
- Pages: 663-668
- First Published: 12 June 2015
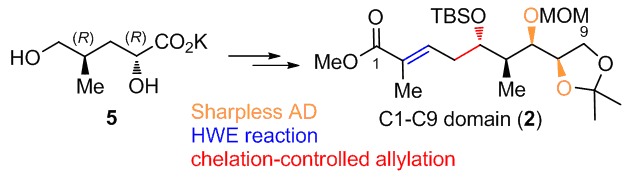
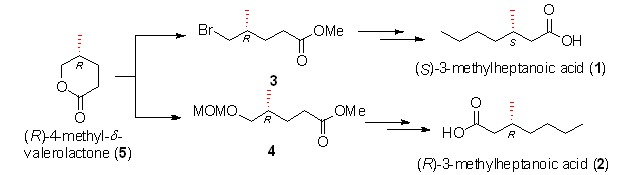
Herein we describe a synthesis of C1–C9 domain 2 of the proposed structure of didemnaketal A, a natural product with important bioactivities, from potassium (2R,4R)-2,5-dihydroxy-4-methylpentanoate 5. Sharpless asymmetric dihydroxylation introduced the chiral vicinal diol and chelation-controlled allylation established another chiral OH.
Semisynthesis of Azedarachol from Pregnanetriol, a Degradative Product of Tigogenin
- Pages: 669-673
- First Published: 10 June 2015
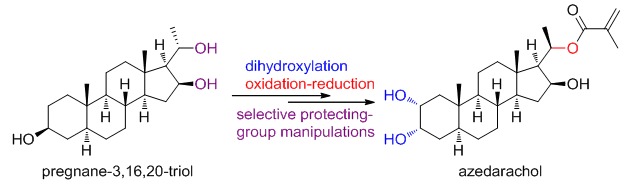
Described herein is a semisynthesis of azedarachol from pregnanetriol, which featured a symbiotic elimination/deprotection process, an oxidation/reduction procedure for reversing C20 configuration, and a dehydration-dihydroxylation process to introduce 2,3-cis-diol. This synthesis also discloses some interesting selectivities of 16,20-diol unit.
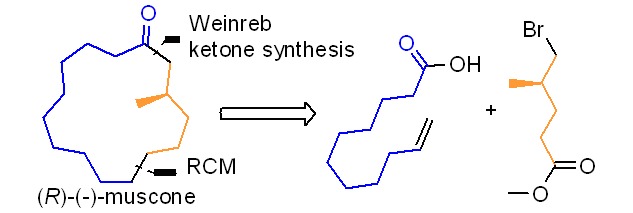
Synthesis of (R)-(−)-Muscone from (R)-5-Bromo-4-methylpentanoate: A Chiron Approach
- Pages: 683-687
- First Published: 09 March 2015
Retraction
Retraction: A Facile Synthetic Route to New Fluorinated Building-Blocks of 1-Fluoroalkynes and 1-Fluorodiynes
- First Published: 17 June 2015