Journal list menu
Export Citations
Download PDFs
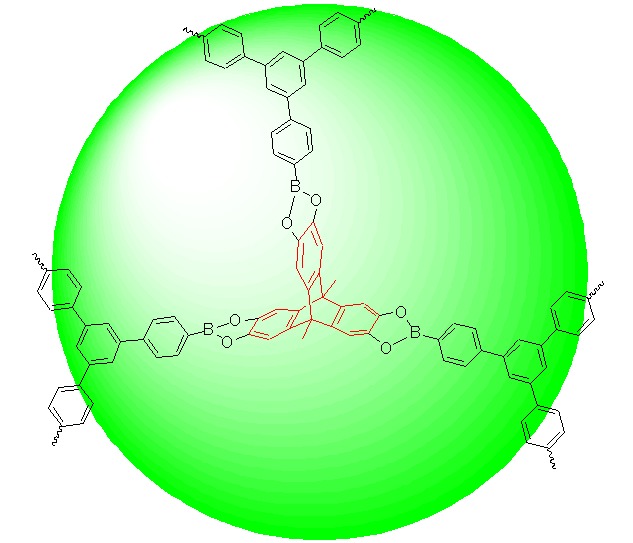
Cover Picture
Cover Picture: A Triptycene-Based Porous Organic Polymer that Exhibited High Hydrogen and Carbon Dioxide Storage Capacities and Excellent CO2/N2 Selectivity (Chin. J. Chem. 5/2015)
- Page: 501
- First Published: 15 May 2015

The cover picture shows a novel porous organic polymer (POP) which was constructed through the condensation of triptycene tricatechol and 1,3,5-benzenetris(4-phenylboronic acid). POPs are a kind of organic materials which possess permanent porosity and thus have great potentials in the field of gas storage and separation. The as-prepared triptycene- based POP exhibits high H2 uptake (up to 1.84 wt% at 77 K, 1 bar), large CO2 adsorption capacity (up to 18.1 wt% at 273 K, 1 bar), and excellent CO2/N2 adsorption selectivity (up to 120/1). The influence of solvent on the gas adsorption performance of the POP has also been investigated. More details are discussed in the article by Zhao et al. on page 539–544.
Contents
Review
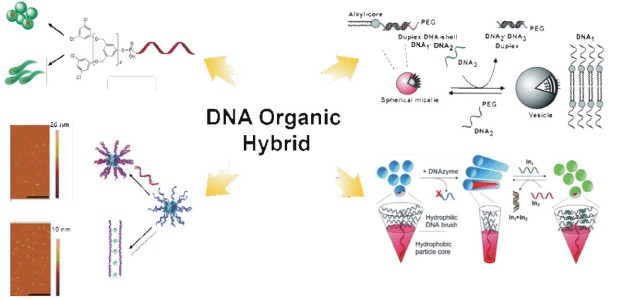
An Overview of Self-Assembly and Morphological Regulation of Amphiphilic DNA Organic Hybrids
- Pages: 511-516
- First Published: 30 March 2015
Communications
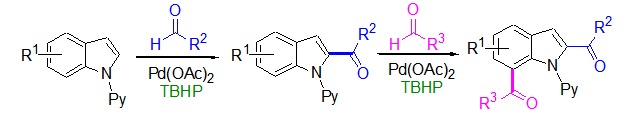
Palladium-Catalyzed Direct C2- and Further C7-Acylation of Indoles with Aldehydes
- Pages: 517-521
- First Published: 05 May 2015
Self-assembly of Micrometer-long DNA Nanoribbons with Four Oligonucleotides
- Pages: 522-526
- First Published: 05 May 2015
Here, we present a protocol for the assembly of DNA nanoribbons with only four oligonucleotides. DNA nanoribbons with different dimensions were successfully assembled with a 96-base scafford strand and three short staples. This approach suggests that there exists a vast design space for the creation of simple nucleic acid nanostructures which could facilitate their application in plasmonic or drug delivery.
A Robust Solution-Based Approach to Monodisperse Hybrid Janus Nanofibers
- Pages: 527-530
- First Published: 05 May 2015
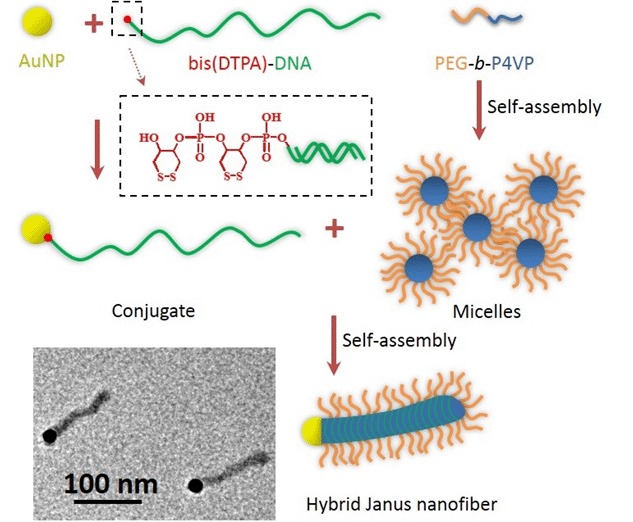
Monodisperse hybrid Janus nanofibers with the structure that one Au nanoparticle (AuNP) is connected to one end of a polymeric nanofiber were prepared by the self-assembly between polymeric micelles and the tadpole-like conjugates resulting from one-to-one complexation of long DNA chains with AuNPs.
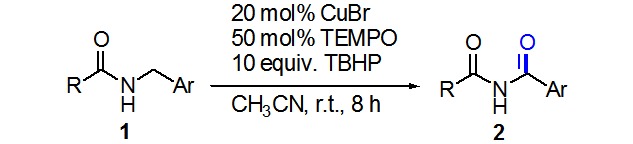
TBHP/TEMPO-Mediated Oxidative Synthesis of Imides from Amides
- Pages: 531-534
- First Published: 05 May 2015
Pd(OAc)2/PPh3-Catalyzed Desulfonylative Homocoupling of Arylsulfonyl Chlorides
- Pages: 535-538
- First Published: 30 March 2015
Full Papers
A Triptycene-Based Porous Organic Polymer that Exhibited High Hydrogen and Carbon Dioxide Storage Capacities and Excellent CO2/N2 Selectivity
- Pages: 539-544
- First Published: 05 May 2015
IBX Oxidation of Benzenedimethanols in the Presence of Cucurbit[8]uril
- Pages: 545-549
- First Published: 30 March 2015
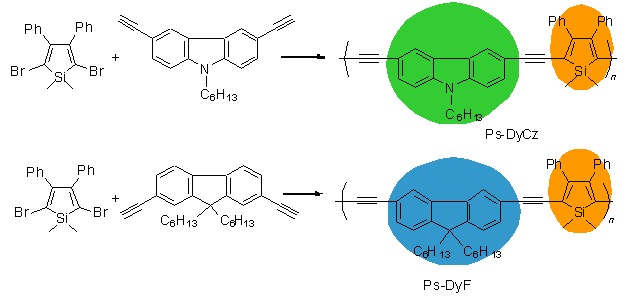
Siloles and Acetenyl Aromatics Copolymers: Synthesis, Characterization and Photophysical Properties
- Pages: 550-558
- First Published: 30 March 2015
Synthesis of Quaternary α-Hydroxy Phosphonates via Direct Hydroxylation of Phosphonate Compounds
- Pages: 559-562
- First Published: 30 March 2015
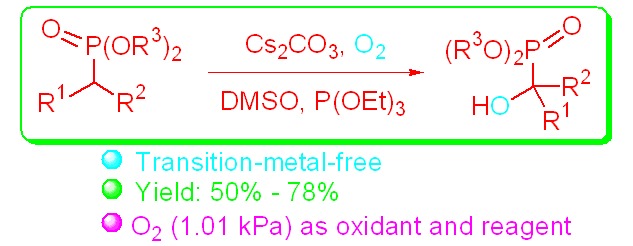
It was found for the first time that Cs2CO3 serves as highly efficient catalyst for the direct hydroxylation reactions of phosphonates under mild conditions. This reaction provides an efficient approach to quaternary α-hydroxy phosphonates, which possess intriguing biological activities and are widely used in many areas.
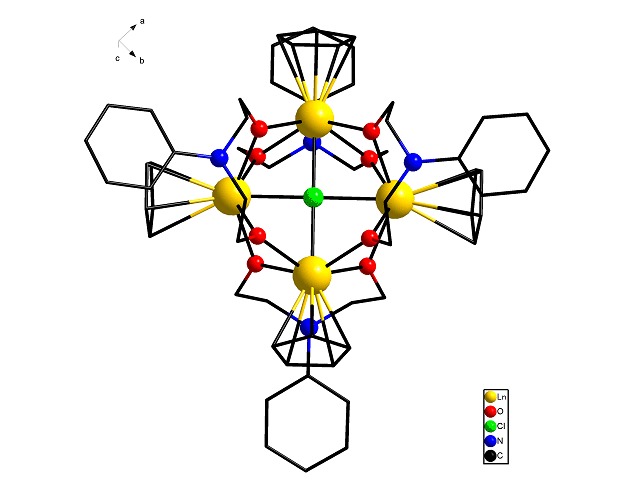
Lanthanocene Diolate Complexes: Synthesis, Structures and Catalytic Property for ε-Caprolactone Polymerization
- Pages: 563-567
- First Published: 09 March 2015
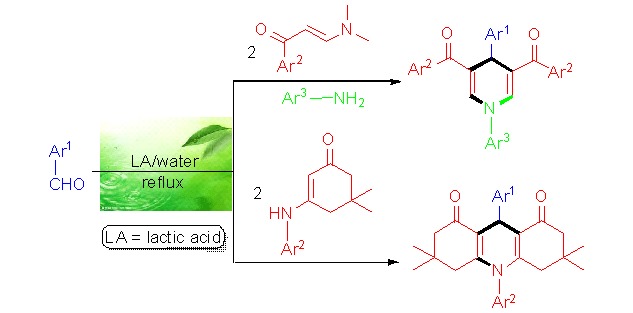
An Environmentally Benign Catalytic Method for Versatile Synthesis of 1,4-Dihydropyridines via Multicomponent Reactions
- Pages: 568-572
- First Published: 05 May 2015
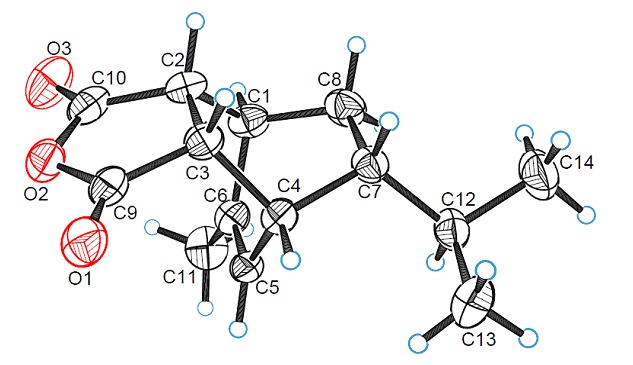
Synthesis and Structure of Tetrahydro-4,7-ethanoisobenzofuran-1,3-dione Derivative
- Pages: 573-577
- First Published: 05 May 2015
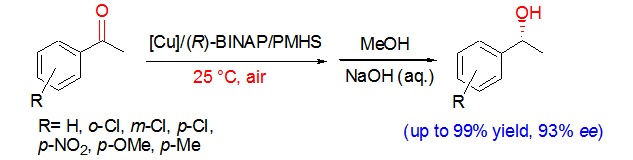
Asymmetric Hydrosilylation of Aromatic Ketones Catalyzed by an Economical and Effective Copper-Diphosphine Catalytic System in Air
- Pages: 578-582
- First Published: 05 May 2015
Effect of Different Ionic Liquids on 5-Hydroxymethylfurfural Preparation from Glucose in DMA over AlCl3: Experimental and Theoretical Study
- Pages: 583-588
- First Published: 30 March 2015
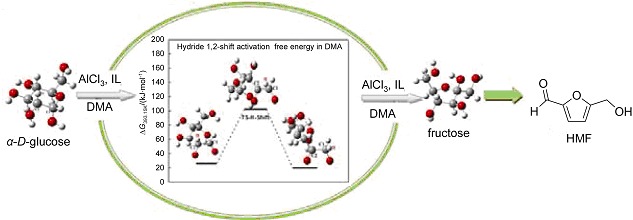
A series of ionic liquids which possess the same cation and different anions were synthesized to explore the effect of anions on 5-hydroxymethylfurfural (HMF) preparation from glucose over AlCl3. Systematic experiments showed that N-methyl-2-pyrrolidone bromide ([NMP]Br) could promote selective conversion of fructose. Theoretical study indicated that Br− could also combine with Al3+, accelerating process of H-shift during glucose isomerization. This discovery can help us choose cocatalysts for low toxic metal, realizing selective conversion of cellulosic biomass conversion.
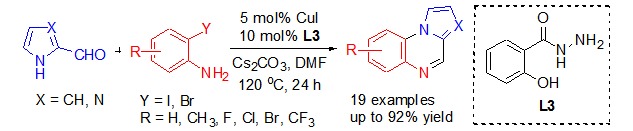
Efficient Copper-Catalyzed Annulation of 2-Formylazoles with 2-Haloanilines for the Synthesis of Pyrrole- and Imidazole-Fused Quinoxalines
- Pages: 589-593
- First Published: 05 May 2015
New Core-Shell Type Polymeric Supports Based on the Amberlite XAD-4 Adsorbent: A Novel Synthesis Procedure
- Pages: 594-600
- First Published: 05 May 2015
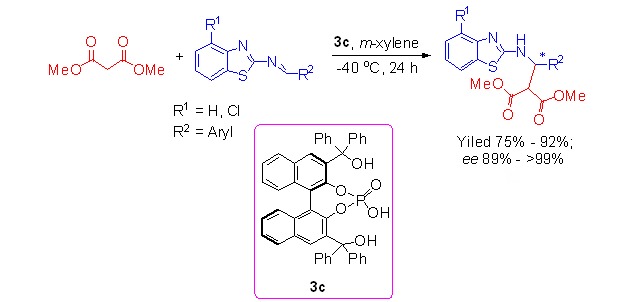
Synthesis, Characterization and Application of Some Axially Chiral Binaphthyl Phosphoric Acids in Asymmetric Mannich Reaction
- Pages: 601-609
- First Published: 05 May 2015
Note
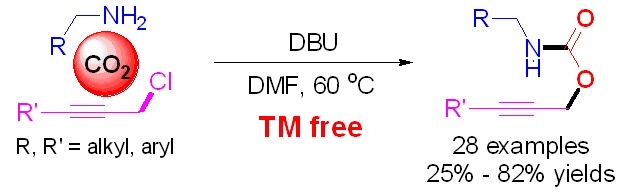
DBU-Mediated Carboxylative Coupling of Primary Amines, Carbon Dioxide and Propargyl Chlorides
- Pages: 610-613
- First Published: 30 March 2015






![IBX Oxidation of Benzenedimethanols in the Presence of Cucurbit[8]uril](/cms/asset/186d0656-ae55-4f50-8dd9-220965efec39/mcontent.jpg)