Journal list menu
Export Citations
Download PDFs
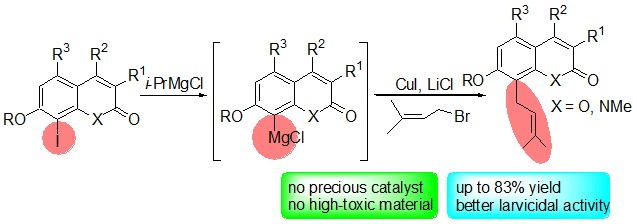
Cover Picture
Cover Picture: Synthesis of Osthole Derivatives with Grignard Reagents and Their Larvicidal Activities on Mosquitoes (Chin. J. Chem. 12/2015)
- Page: 1325
- First Published: 18 December 2015

The cover picture showsan inexpensive and eco-friendly method to synthesize osthole derivatives with Grignard reagents assisted by CuI and LiCl. With the purpose of discovering novel insecticides from Traditional Chinese Medicine (TCM), the structure of osthole has been modified to improve its larvicidal activity against mosquitoes. Starting from Grignard reagents generated from 8-iodocoumarins, a broad range of osthole derivatives can be prepared in mild to good yields (up to 83%). Bio-activity evaluation showed that several products exhibited better larvicidal activities than osthole. More details are discussed in the article by Li et al. on page 1353–1358.
Contents
Contents: Chin. J. Chem. 12/2015
- Pages: 1327-1330
- First Published: 18 December 2015
Communication
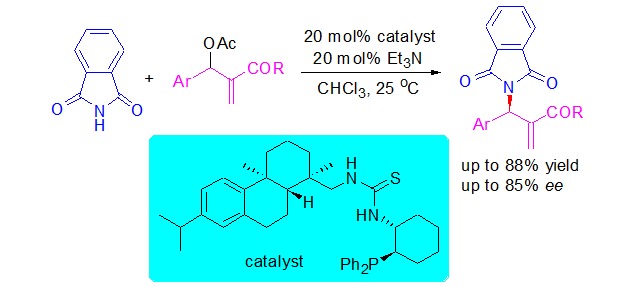
Enantioselective Allylic Amination of Morita-Baylis-Hillman Acetates Catalyzed by Chiral Thiourea-Phosphine
- Pages: 1333-1337
- First Published: 15 December 2015
Full Papers
Temperature-Induced Transformation from Large Compound Vesicles to Worm-like Aggregates by ABC Triblock Copolymer
- Pages: 1338-1346
- First Published: 15 December 2015
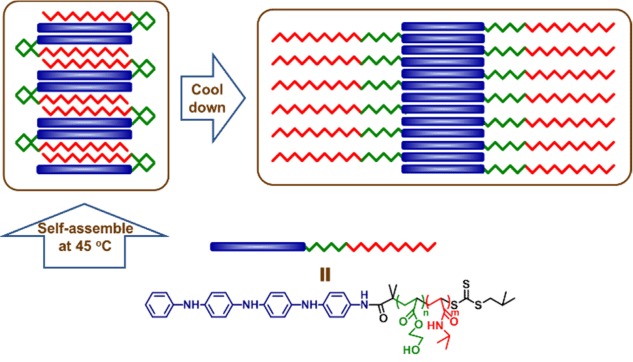
Two triblock polymers, TA-b-PNIPAM-b-PHEA and TA-b-PHEA-b-PNIPAM, were synthesized with identical chemical compositions but different connection order. Both of their aggregates have spherical shape assembled at 45°C. However, when their aggregate dispersion was cooled down to 20°C, only TA-b-PHEA-b-PNIPAM's morphology changed, forming worm-like aggregates with the diameter of about 100–200 nm.
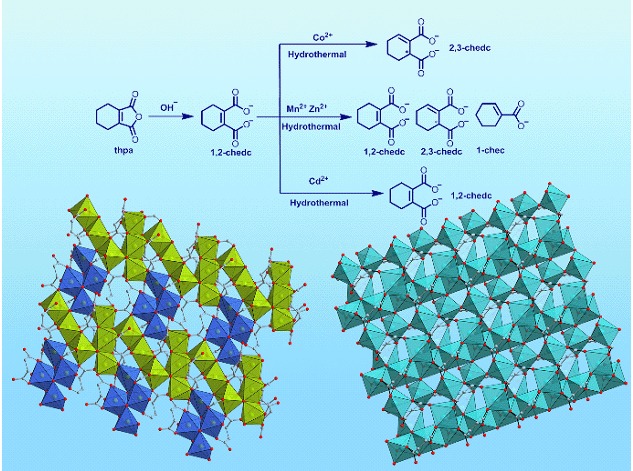
Influence of the Metal Ions on the Allylic Rearrangement Reaction of 3,4,5,6-Tetrahydrophthalic Anhydride
- Pages: 1347-1352
- First Published: 15 December 2015
Synthesis of Osthole Derivatives with Grignard Reagents and Their Larvicidal Activities on Mosquitoes
- Pages: 1353-1358
- First Published: 15 December 2015
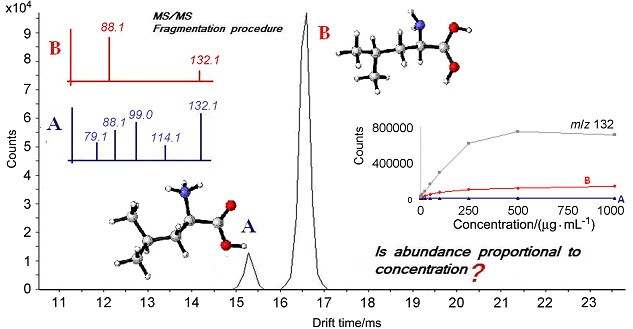
Behaviors of Leucine and Isoleucine in Ion Mobility-Quadrupole Time of Flight Mass Spectrometry
- Pages: 1359-1364
- First Published: 13 November 2015
Study on the Degradation of the Highly Reactive Hypervalent Trifluoromethylation Iodine Reagent PhI(OAc)(CF3)
- Pages: 1365-1370
- First Published: 15 December 2015
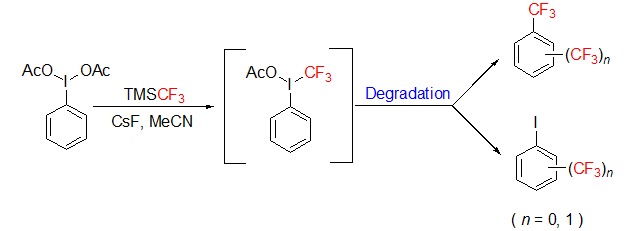
Degradation of the highly reactive hypervalent trifluoromethylation iodine reagent PhI(OAc)(CF3), which can only be generated in situ with mixing PhI(OAc)2 and TMSCF3 in the presence of CsF, was studied by ESI-MS and GC-MS combined with 19F-NMR. The important transient intermediate PhICF3+ was determined by ESI-MS, and the major volatile products containing CF3 were identified with the authentic compounds by using GC-MS, such as trifluoromethylbenzene, 2-iodobenzotrifluoride, 3-iodobenzotrifluoride, 4-iodobenzotrifluoride. Meanwhile, more evidences obtained with 19F-NMR were given for such degradation reaction. A possible rapid CF3 radical transfer reaction pathway was proposed to clarify such degradation progress based on the experimental results. Therefore, this study may be helpful in elucidating the intrinsic reactivity of PhI(OAc)(CF3) and the possible competing side reactions caused by such self-degradation pathway.
Three-Component Reaction for Construction of Spiro[indoline-3,7′-thiazolo[3,2-a]pyridines] and Spiro[benzo[4,5]thiazolo[3,2-a]pyridine-3,3′-indolines]
- Pages: 1371-1379
- First Published: 13 November 2015
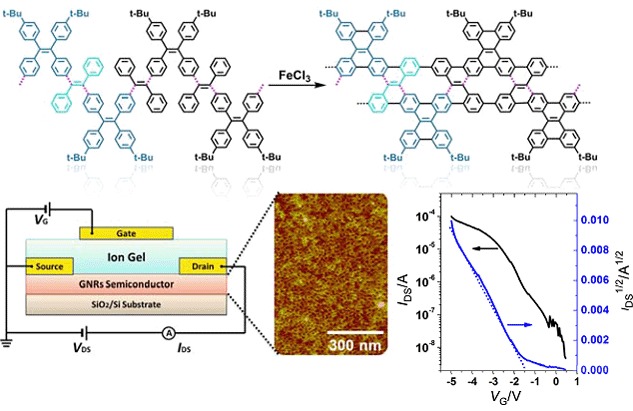
Graphene Nanoribbons from Tetraphenylethene-Based Polymeric Precursor: Chemical Synthesis and Application in Thin-Film Field-Effect Transistor
- Pages: 1380-1388
- First Published: 15 December 2015
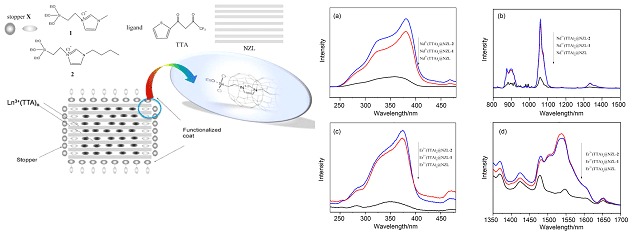
Enhanced NIR Luminescence of Nanozeolite L Loading Lanthanide β-Diketonate Complexes
- Pages: 1389-1392
- First Published: 18 December 2015
Note
Synthesis and Catalytic Study of Ruthenium Carbene Catalyst Containing a Zn-Porphyrin Ligand
- Pages: 1393-1397
- First Published: 15 December 2015
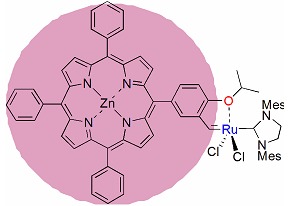
A ruthenium carbene complex containing a Zn-porphyrin ligand was synthesized. It was characterized by 1H NMR, IR, HRMS and elemental analysis. The activity of the complex for ring-closing metathesis and cross-metathesis reactions was investigated. The complex exhibited high catalytic activity for many different olefin substrates.





![Three-Component Reaction for Construction of Spiro[indoline-3,7′-thiazolo[3,2-a]pyridines] and Spiro[benzo[4,5]thiazolo[3,2-a]pyridine-3,3′-indolines]](/cms/asset/eb55f04e-c158-4ef0-99a1-4646546f540b/mcontent.jpg)