Journal list menu
Export Citations
Download PDFs
Cover Picture
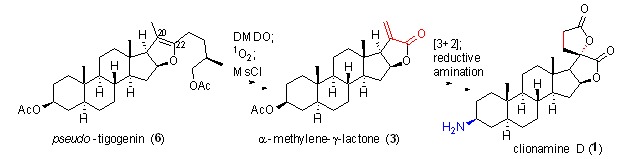
Cover Picture: A Short Synthesis of Clionamine D (Chin. J. Chem. 11/2015)
- Page: 1221
- First Published: 17 November 2015

The cover picture shows a short and efficient synthesis of clionamine D. Steroidal α-methylene-γ-lactones are versatile intermediates for synthesizing related natural products such as clionamines A–D, a family of marine natural alkaloids with potent autophagy bioactivities and unprecedented chemical structures. Employing single oxygen to break the C22-C23 double bond via a [2+2]/retro-[2+2] process, Shi and Tian et al. have developed a scalable, four-step procedure to prepare α-methylene-γ-lactone directly from steroidal sapogenin–needn't prepare dinorcholanic lactone first. A synthesis of clionamine D was therefore achieved in eight steps with an overall yield of 31%. More details are discussed in the article by Tian et al. on page 1235–1238.
Contents
Contents: Chin. J. Chem. 11/2015
- Pages: 1223-1227
- First Published: 17 November 2015
Communications
Preparation, Crystal Structure and Properties of a New Crystal Form of Diammonium 5,5′-bistetrazole-1,1′-diolate
- Pages: 1229-1234
- First Published: 28 October 2015
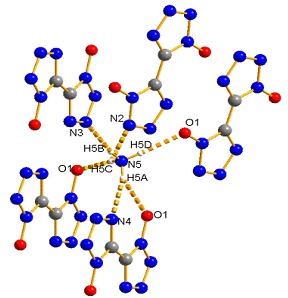
A new crystal form of diammonium 5,5′-bistetrazole-1,1′-diolate (1) was prepared by two different novel methods and found as monoclinic and space group of P21/c (14). The thermal decomposition analysis and sensitivities test towards impact, friction of 1 indicated that 1 has much lower sensitivities than those of RDX/HMX and comparable to those of TNT, which suggested that 1 could be used as a good candidate of new insensitive energetic compound.
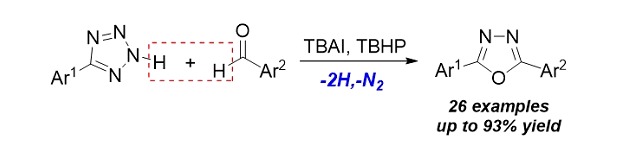
Organocatalytic Oxidative Amidation of Aldehydes with Tetrazoles to Construct 2,5-Diaryl 1,3,4-Oxadiazoles
- Pages: 1239-1243
- First Published: 28 October 2015
Full Papers
Efficient One-Pot Access to 2,9-Dihydrothiopyrano[2,3-b]indole Scaffolds Showing Large Stokes Shifts
- Pages: 1244-1250
- First Published: 28 October 2015
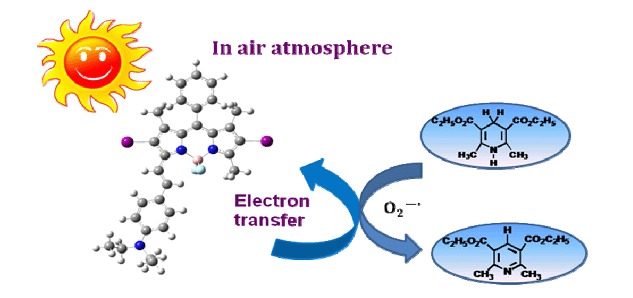
Intramolecular Charge Transfer-Enhanced BODIPY Photosensitizer in Photoinduced Electron Transfer and Its Application to Photoxidation under Mild Condition
- Pages: 1251-1258
- First Published: 28 October 2015
Cyanate Ester/Functionalized Silica Nanocomposite: Synthesis, Characterization and Properties
- Pages: 1259-1268
- First Published: 28 October 2015
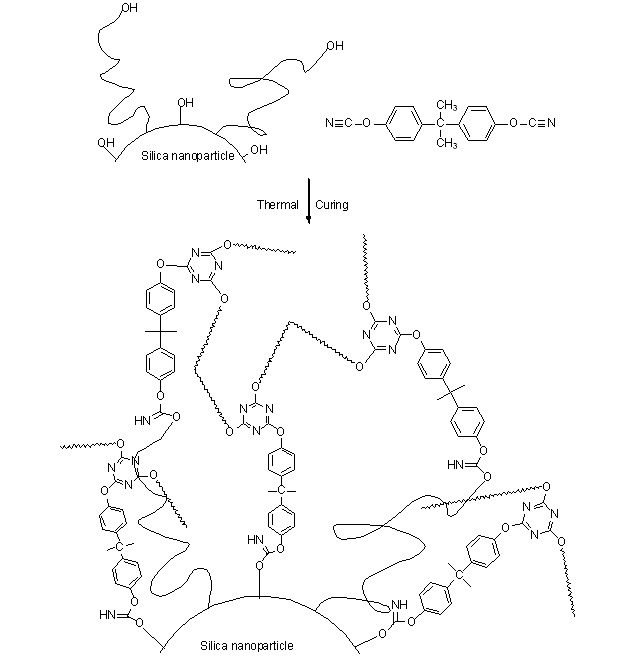
A novel functionalized silica nanocomposite (F-SiO2), with 5-isocyanato-1-isocyanatomethyl-1,3,3-trimethylcyclohexane (IPDI) acting as a linking agent to connect hydroxyl-terminated polybutadiene (HTPB) and silica, was prepared to modify the bisphenol A dicyanate ester (BADCy). The incorporation of appropriate content of modified F-SiO2 can enhance the mechanical properties of BADCy resin. In addition, the thermal stability of BADCy/F-SiO2 nanocomposties is also superior to that of pure BADCy resin.
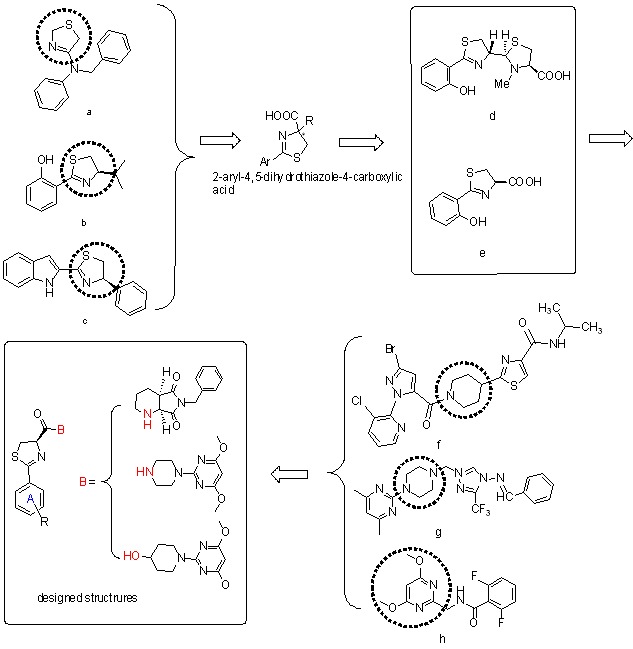
Design, Synthesis, Antifungal Activities and SARs of (R)-2-Aryl-4,5-dihydrothiazole-4-carboxylic Acid Derivatives
- Pages: 1269-1275
- First Published: 13 November 2015
Synthesis and Acid-Catalyzed Cyclization of 2-Alkenylstilbenes: a New Approach to the Substituted Indenes
- Pages: 1276-1286
- First Published: 13 November 2015
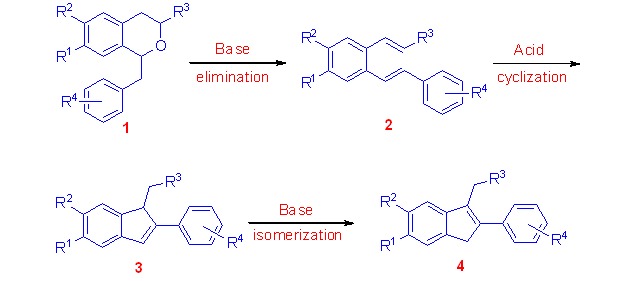
A base-catalyzed ring-opening of 1-benzylisochromans 1 firstly produced 2-alkenylstilbenes 2, which then underwent a mild acid-catalyzed intramolecular cyclization to furnish 1,2-disubstituted indenes 3 in high yields. Subsequently, a base-catalyzed isomerization of the 1,2-disubstituted indenes 3 afforded the more stable 2,3-disubstituted indenes 4 in almost quantitative yields.
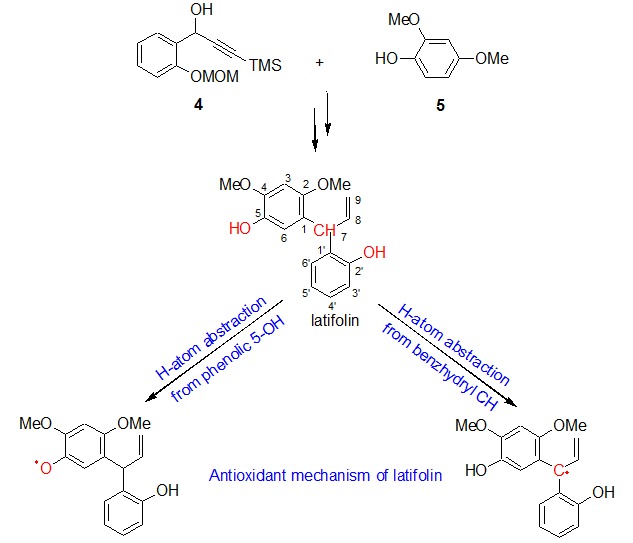
First Total Synthesis of (±)-Latifolin and Its Antioxidant Mechanism
- Pages: 1287-1292
- First Published: 29 September 2015
Fabrication of Porous Nitrogen-Doped Carbon Materials as Anodes for High-Performance Lithium Ion Batteries
- Pages: 1293-1302
- First Published: 13 November 2015
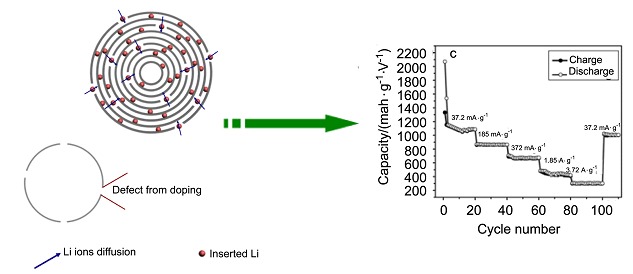
A porous nitrogen-doped carbon material was fabricated by using nitrogen containing gelatin as the carbon source and nano-silica obtained by a simple flame synthesis approach as the template. The as-prepared carbons (especially the HNC-700) delivered optimal reversible capacities of 1084 mAh·g−1 at the current density of 37.2 mA·g−1 (0.1 C) and 309 mAh·g−1 even at 3.72 A·g−1 (10 C). These results suggest that the as-obtained carbon materials would be promising anode materials for lithium ion batteries.
Preparation and Electrochemical Performance of Li[Ni1/3Co1/3Mn1/3]O2 Synthesized Using Li2CO3 as Template
- Pages: 1303-1309
- First Published: 13 November 2015
![Preparation and Electrochemical Performance of Li[Ni1/3Co1/3Mn1/3]O2 Synthesized Using Li2CO3 as Template](/cms/asset/48956628-0069-45ce-b1d1-273451ae29be/mcontent.jpg)
The porous Li[Ni1/3Co1/3Mn1/3]O2 has been synthesized via a facile carbonate co-precipitation method using Li2CO3 as template and lithium-source. The porous structure of Li[Ni1/3Co1/3Mn1/3]O2 can offer more Li+ location and shorten the distance of Li+ ion and electron, resulting in excellent electrochemical performance.
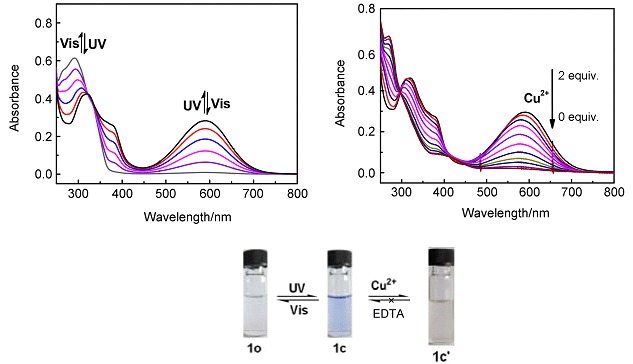
A Highly Selective Chemosensor for Cu2+ Based on a Diarylethene Linking an Aminoquinoline Unit
- Pages: 1310-1316
- First Published: 13 November 2015
Note
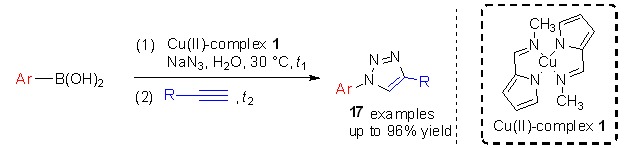
An Efficient Copper-Catalyzed One-Pot Synthesis of 1-Aryl-1,2,3-triazoles from Arylboronic Acids in Water under Mild Conditions
- Pages: 1317-1320
- First Published: 13 November 2015





![Efficient One-Pot Access to 2,9-Dihydrothiopyrano[2,3-b]indole Scaffolds Showing Large Stokes Shifts](/cms/asset/7a9c6b34-0552-4f55-baa1-5a24bd4997c4/mcontent.jpg)