Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture: Foldamer-Derived Preorganized Bi- and Tri-zinc Porphyrin Tweezers for a Pentafluorobenzene-bearing Pyridine Guest: The Binding Pattern Study (Chin. J. Chem. 5/2013)
- Page: 557
- First Published: 22 May 2013

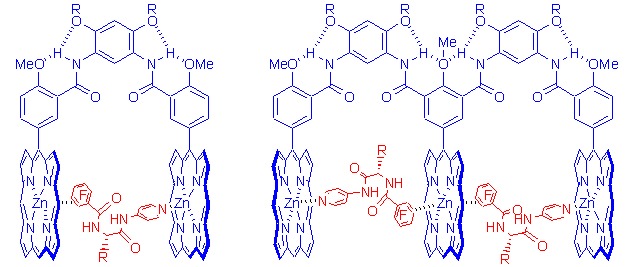
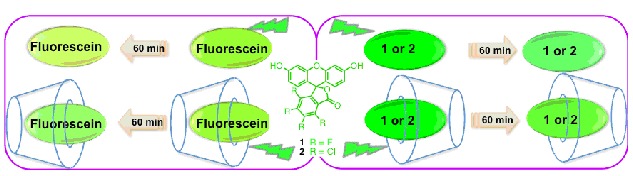
The cover picture shows the binding gradient effect of two hydrogen bonding-driven foldamer-dirived molecular tweezers. For a pentafluorophenyl-appended pyridine ligand, in the cavity of the tweezer, two binding interactions, i.e., the N-Zn(II) coordination and zinc porphyrin-pentafluorobenzene donor-acceptor interactions stabilize the encapsulation of the ligand in the cavity of the tweezers. After the binding in the cavity take places, further coordination of the ligand from outside the tweezer becomes much less favorable due to the interaction occurring in the cavity, which is evidenced by the UV-vis and 1H and 19F NMR experiments. More details are discussed in the article by Li et al. on page 582–588.
Editorial
Contents
Review
Cyclodextrin-Based Porous Nanocapsules
- Pages: 569-576
- First Published: 21 May 2013
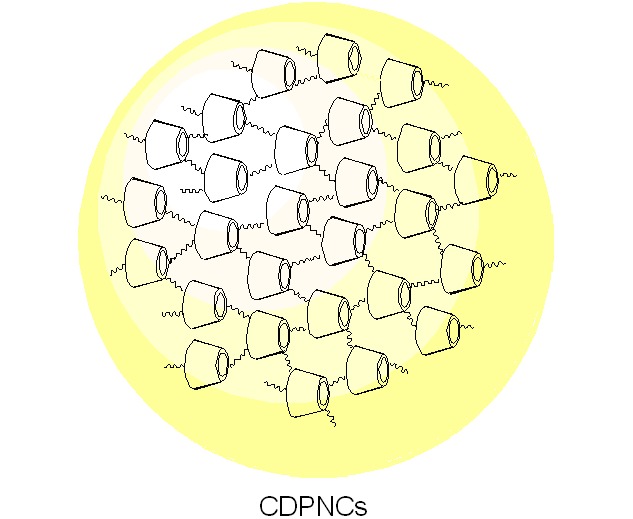
CD-based porous nanocapsules (CDPNCs) are a class of covalently cross-linked networks based on CDs with a three-dimensional structure. Chemical cross-linking is an important way developed to prepare CDPNCs. CDPNCs can be obtained using different cross-linkers and preparation methods, such as homogeneous method, miniemulsion polymerization, and emulsion-solvent evaporation method. Because of cross-linking, a three-dimensional network is built which forms the porous structure in CDPNCs with the cavities of CDs. CDPNCs not only take on the merits of CDs, including host-guest interaction, molecular recognition, stability, and biocompatibility, but also possess new functions given by the porous structure. CDPNCs are promising nanomaterials which are expected to be applied in diverse fields.
Communication
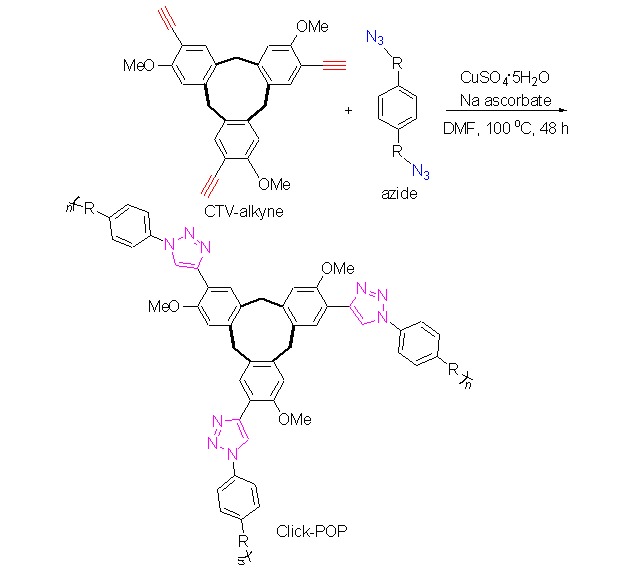
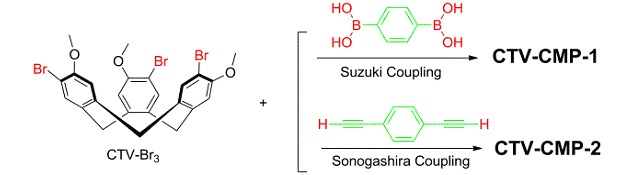
Synthesis and Properties of Porous Organic Polymers from a Rigid Macrocyclic Building Block
- Pages: 577-581
- First Published: 14 May 2013
Full Papers
Foldamer-Derived Preorganized Bi- and Tri-zinc Porphyrin Tweezers for a Pentafluorobenzene-bearing Pyridine Guest: The Binding Pattern Study
- Pages: 582-588
- First Published: 06 March 2013
Synthesis, Structure and Coordination Self-Assembly of Azacalix[4-n]pyridine[n]pyrazines (n=1–3)
- Pages: 589-597
- First Published: 03 April 2013
![Synthesis, Structure and Coordination Self-Assembly of Azacalix[4-n]pyridine[n]pyrazines (n=1–3)](/cms/asset/1878a5c3-dd22-4b0e-8e74-081da25cfd39/mcontent.jpg)
Three new macrocyclic azacalix[4]aromatics that comprise various numbers of pyridine and pyrazine rings bridged by N-CH3 groups were synthesized. Structural characterization explored that their conformations are fluxional in solution and each adopts a 1,3-alternate configuration in crystalline solids.
Selectively Fluorescent Sensing Behavior of Phenylaza-15-crown-5-triazolyl Coumarin for Hg2+ and Fe3+ in Alcohol and Aqueous Media Respectively
- Pages: 598-602
- First Published: 14 May 2013
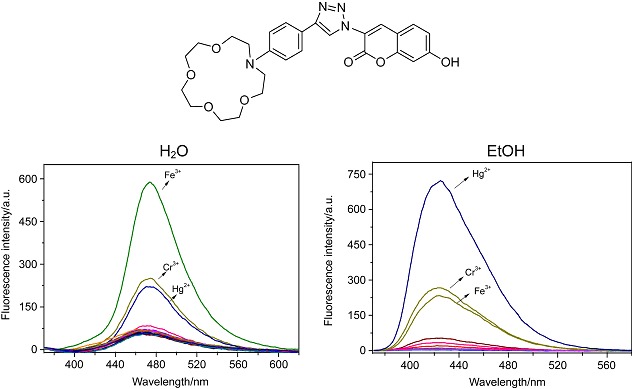
A π-conjugated phenylaza-15-crown-5-triazol-substituted coumarin fluoroionophore 1 was synthesized. 1 can display selective fluorescence enhancement toward Fe3+ over Hg2+, Cr3+ and the other metal ions in aqueous solution. In sharp contrast, Hg2+ gives the largest fluorescence enhancement over Cr3+, Fe3+ and the other metal ions in EtOH.
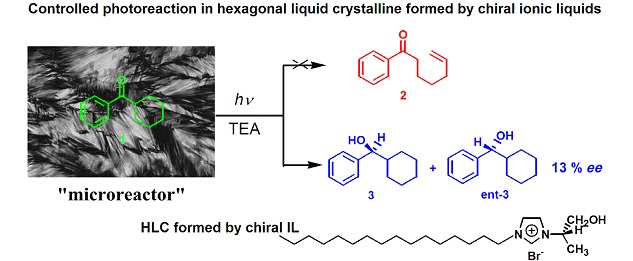
Stereoselective Photochemical Reaction of Cyclohexyl Phenyl Ketone within Lytropic Liquid Crystals Formed by Chiral Ionic Liquids
- Pages: 603-606
- First Published: 14 May 2013
Complexation of Triptycene-Derived Macrotricyclic Host with Bisparaquat Derivative and Self-Folding Guest: A Switchable Process Controlled by K+ Ions
- Pages: 607-611
- First Published: 29 April 2013
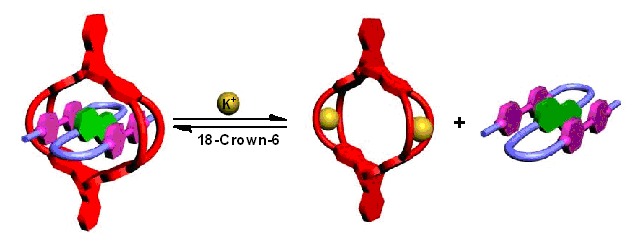
Triptycene-derived cylindrical macrotricyclic host with a large central cavity could form stable 1:1 complexes with not only polyether linked bisparaquat derivative but also a self-folding A-D-A guest. Moreover, switchable processes between the host and both the bisparaquat derivative and the self-folding guest could be controlled by potassium ions.
One-Pot Synthesis of Tetrafluoro- and Tetrachlorofluorescein Derivatives and Their Stabilization by β-Cyclodextrin
- Pages: 612-616
- First Published: 29 April 2013
Conjugated Porous Networks Based on Cyclotriveratrylene Building Block for Hydrogen Adsorption
- Pages: 617-623
- First Published: 14 May 2013
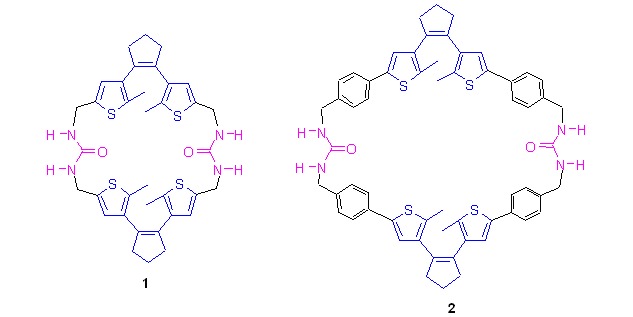
Complexation Selectivities of Pillar[5]arenes with Primary Ammonium Salts
- Pages: 624-626
- First Published: 14 May 2013
Novel Macrocycles Bearing Dithienylethene Units and Urea Functional Groups: Synthesis, Structure and Photochromic Property
- Pages: 627-634
- First Published: 14 May 2013
New Crystalline Forms of Mebendazole with n-Alkyl Carboxylic Acids: Neutral and Ionic Status
- Pages: 635-640
- First Published: 14 May 2013
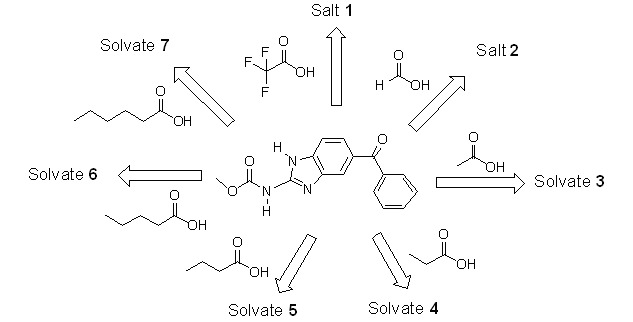
The cocrystallization of mebendazole with a series of n-alkyl carboxylic acids generated two salts of 1–2 and five solvates of 3–7, and their structures and thermal stabilities were investigated. Compounds 1–7 provide new solid forms of mebendazole for improvement of its physical and chemical properties.
Multichannel Chromogenic and Chiral Anions Recognition by Imidazolium Functionalized BINOL Derivatives
- Pages: 641-650
- First Published: 29 April 2013
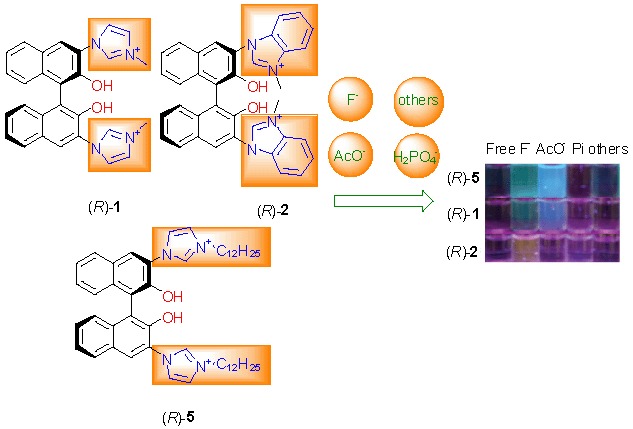
An effective strategy to create anions probes by conjugating imidazolium with BINOL derivatives has been achieved, and this method enabled naked eye and dual channel (absorption and fluorescence) detection of F− and AcO− ions. More importantly, introduction of a lipophilic dodecyl appendage at imidazolium nitrogen ((R)-5) led to dramatic enhancement of the interaction with anions.
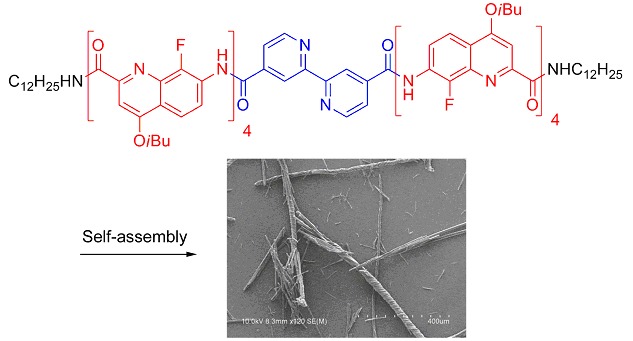
Twisted Helical Microfibers by Hierarchical Self-Assembly of an Aromatic Oligoamide Foldamer
- Pages: 651-656
- First Published: 14 May 2013
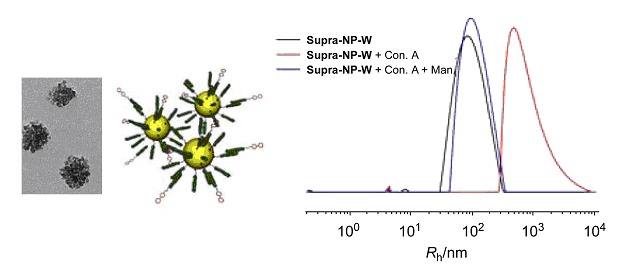
Preparation of Tris(2-aminoethyl)amine-Cross-Linked Cyclodextrin-Based Porous Nanospheres and Their Application as Drug Delivery Systems
- First Published: 21 May 2013
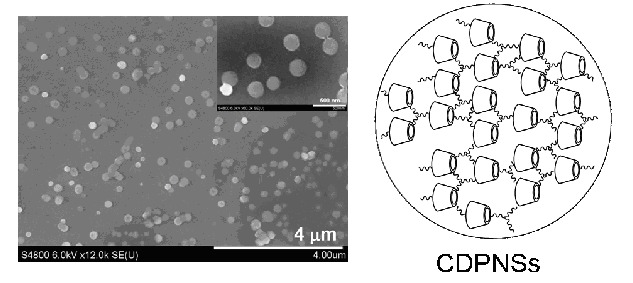
A green, simple, and effective approach was developed to synthesize biocompatible cyclodextrin-based porous nanospheres (CDPNSs). The cross-linking is allowed to take place at room temperature in N,N-dimethylformamide (DMF) with tris(2-aminoethyl)amine (TAA) as the cross-linker. The resulting CD-based polymers (CDPs) were reshaped from a random morphology into a spherical shape during a dialysis process, and CDPNSs were formed with the average diameter of 237±93 nm and the size distribution of 100–400 nm. Furthermore, a study on drug loading and in vitro release behavior of CDPNSs was carried out, where acridine red (AR) was chosen as a drug model. The loading capacity is up to (9.8±0.2) wt% and the encapsulation efficiency is up to (40.2±0.8) wt%. With regard to the release of AR from CDPNSs in vitro, (82.3±0.2)% of AR was released within 24 h. It is expected that CDPNSs will find potential applications as drug delivery systems in the future.
Self-assembly of Pyrene-modified Rhomboidal Metallodendrimers via Directional Metal-ligand Bonding Approach
- Pages: 663-672
- First Published: 14 May 2013
Synthesis of Bis-benzimidazolium Cyclic Receptors and Their Anion Binding Properties
- Pages: 673-678
- First Published: 01 March 2013
Sulfate Binding with a Tripodal Tris(4-pyridylurea) Receptor
- Pages: 679-683
- First Published: 21 May 2013
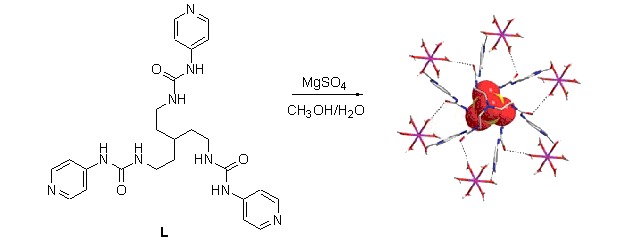
The tripodal tris(4-pyridylurea) receptor (L) was synthesized and its anion binding properties studied. The ligand forms a 2:1 (H/G) complex with MgSO4, [SO42−⊂L2], in which a sulfate ion is encapsulated by six urea groups as in the analogous complex of the 3-pyridyl-substituted ligand. The anion binding behavior of ligand L in solution was studied.
Tetraamido-oxacalix[4]arene Derivatives: Synthesis, Structures and Supramolecular Assemblies
- Pages: 684-688
- First Published: 03 April 2013
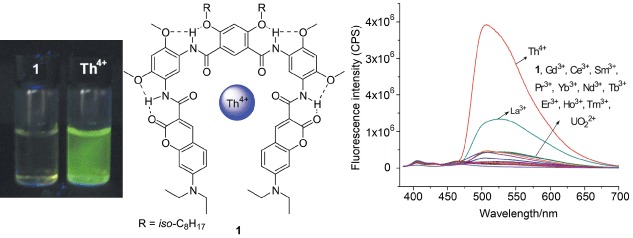
Highly Selective Fluorescent Recognition towards Th4+ Based on Coumarin-derivatized Crescent Aromatic Oligoamide
- Pages: 689-694
- First Published: 21 May 2013
Non-covalent Sugar Modification and Self-assembly of Fluorous Gold Nanoparticles Driven by Fluorous Interaction
- Pages: 695-700
- First Published: 21 May 2013





![Complexation Selectivities of Pillar[5]arenes with Primary Ammonium Salts](/cms/asset/b32959c0-0c03-44a9-9536-e0e8b7f5a8cc/mcontent.jpg)
![Tetraamido-oxacalix[4]arene Derivatives: Synthesis, Structures and Supramolecular Assemblies](/cms/asset/1b176fbe-cd54-4ac8-a748-dd4d4a76c1a2/mcontent.jpg)