Journal list menu
Export Citations
Download PDFs
ISSUE INFORMATION
PERSPECTIVE
Increased risk of cancer in dogs and humans: A consequence of recent extension of lifespan beyond evolutionarily determined limitations?
- Pages: 3-19
- First Published: 23 February 2022
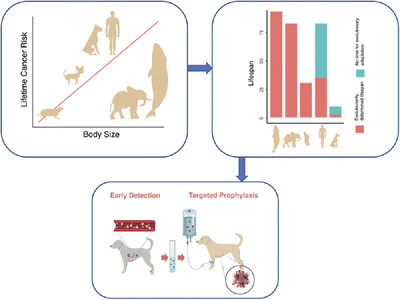
Peto's paradox states that, at the species level, cancer incidence is not correlated with the number of cells in an organism. Furthering the paradox, cancer incidence is also not correlated with longevity among different species. One explanation for the relatively low cancer rates in large and/or long-lived species, exemplified here by bowhead whales, African elephants, and African naked mole rats, is the evolution of tumor suppressive mechanisms, unique to each species, over millions of years. In contrast to these species, cancer is among the most common causes of death in modern dogs and in modern humans. Both of these species share a recent, dramatic increase in life expectancy driven by breakthroughs in medicine and technology, and without concurrent evolutionary adaptations that can protect individuals from cancer in the years of life “beyond what nature intended.” Thus, a reasonable approach to address the apparent cancer epidemic would be to prevent the disease entirely. We are developing tests for early cancer detection and risk stratification in dogs, paired with targeted prophylaxis, with the goal of preventing or delaying the emergence of cancer. This proof-of-concept in dogs would set a path for development of comparable approaches in humans.
REVIEWS
The critical function of metabolic reprogramming in cancer metastasis
- Pages: 20-43
- First Published: 23 February 2022
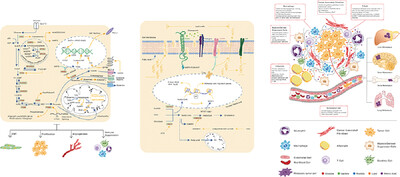
Metabolism alterations of glucose, lipid, and amino acid provide cancer cells with energy and substances for metastasis. Meanwhile, non-tumor cells in TME also undergo metabolic reprogramming or respond to metabolites to promote migration and invasion of cancer cells. Understanding metabolic reprogramming during cancer metastasis is helpful for developing new anti-tumor strategy.
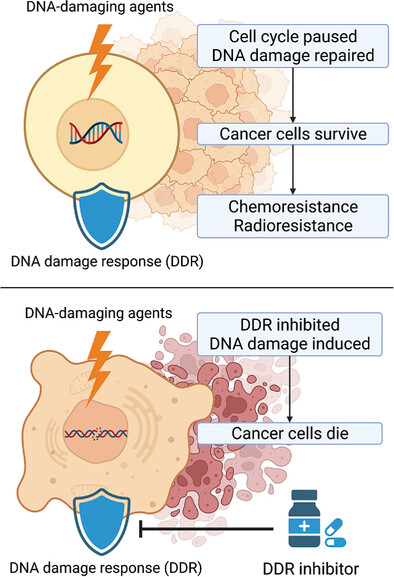
DNA damage response inhibition-based combination therapies in cancer treatment: Recent advances and future directions
- Pages: 44-67
- First Published: 07 March 2022
ORIGINAL ARTICLE
Immune checkpoint expression and relationships to anti-PD-L1 immune checkpoint blockade cancer immunotherapy efficacy in aged versus young mice
- Pages: 68-83
- First Published: 25 February 2022
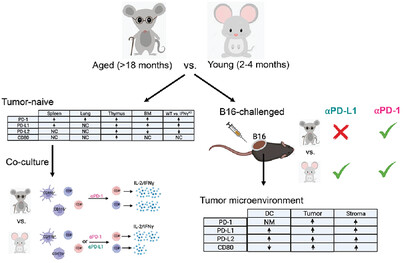
Immune checkpoint expression can influence cancer response to immune checkpoint inhibitors. Garcia, et al., show that immune, tumor and stromal expression of distinct IC is higher in aged versus young and such changes could affect differential response to αPD-1 vs αPD-L1 immune checkpoint inhibitors. Notably, αPD-1 increase polyfunctional (IFN-γ/IL-2-producing) CD8+ and CD4+ T cells in aged whereas both αPD-1 and αPD-L1 increased both CD4+ and CD8+ polyfunctional T cells in young. (NC, no change; NM, not measured, ⨯ = not effective, ✓ = effective).