The critical function of metabolic reprogramming in cancer metastasis
Sun-Zhe Xie, Jun-Jie Pan, and Jian-Feng Xu contributed equally to this work.
Abstract
Cancer metastasis is the leading cause of cancer-related death. It is a complex, inefficient, and multistep process related to poor prognosis and high mortality of patients. Increasing evidence has shown that metabolic programming is a recognized hallmarker of cancer, plays a critical role in cancer metastasis. Metabolism alterations of glucose, lipid, and amino acid provide cancer cells with energy and substances for biosynthesis, maintain biofunctions and significantly affect proliferation, invasion, and metastasis of cancer cells. Tumor microenvironment (TME) is a complex system formed by varieties of cellular and noncellular elements. Nontumor cells in TME also undergo metabolic reprogramming or respond to metabolites to promote migration and invasion of cancer cells. A comprehensive understanding of the regulatory mechanism in metastasis from the metabolic reprogramming aspect is required to develop new therapeutic strategies combatting cancer metastasis. This review illustrates the metabolic reprogramming and interaction of cancer cells and nontumor cells in the TME, and the development of treatment strategies targeting metabolism alterations.
1 INTRODUCTION
In the past decades, significant advances in cancer prevention and treatment measures have been obtained.1 However, the cancer burden remains high worldwide, and over 90% of patients with solid cancers die of cancer metastasis. Metastasis is a process of cancer cells disseminating from the primary sites and outgrowing in distant organs. Cancer metastasis is a complex and dynamic cascade of multiple parallel overlapping steps, which includes invading into the circulatory system, surviving during hematogenous transit, penetrating through vascular walls into the parenchyma of distant target organ, forming dormant- or micrometastatic colonies, and finally proliferating into clinically detectable metastatic lesions.2, 3 Cancer cells undergo complex biological changes in the metastatic cascade, making them adapt to the unfavorable microenvironment. However, opposite to the traditional linear route that metastasis may occur in the late stage of tumors according to the clinical manifestation of metastasis, increasing evidence shows that dissemination of cancer cells from primary to distant sites often occur much earlier, even before the clinical detection and diagnosis of primary tumor.4, 5 Although many cancer cells enter the metastatic cascade, only a few cells can reach remote organs and subsequently proliferate into micrometastatic colonies, hinting the high inefficiency of metastasis.
As a respond to nutritionally deficient environments, tumor cells undergo significant switch in a series of critical metabolic mode to meet the needs of biological function, called “metabolic reprogramming.”6 Metabolic reprogramming is recognized as one of the most important characteristics of tumor cells.7 In 1889, the “seed and soil” hypothesis was proposed by Stephen Paget, who believed the tumor microenvironment (TME) is the “soil” on which the “seeds” (tumor cells) depend for survival.8 Following research also showed the formation of metastases requires the coordination between “seeds” and “soil.”9 The TME is a complex system composed of stromal cells, inflammatory immune cells, vasculature, and extracellular matrix (ECM).10 The TME acts as a scaffold and has great effects on pro- and antitumor metastasis. Accumulating evidence shows that a variety of cells in TME also undergo metabolic reprogramming. To a certain extent, the metabolic interplay between nontumor cells and tumor cells fuels tumor metastasis.11 In this review, we discuss the metabolic reprogramming of glucose, lipid, amino acid in cancer cells and their interactions with the nontumor cells in TME from metabolic aspects. Moreover, we also briefly show the development of promising therapeutic strategies targeting cancer metabolic reprogramming.
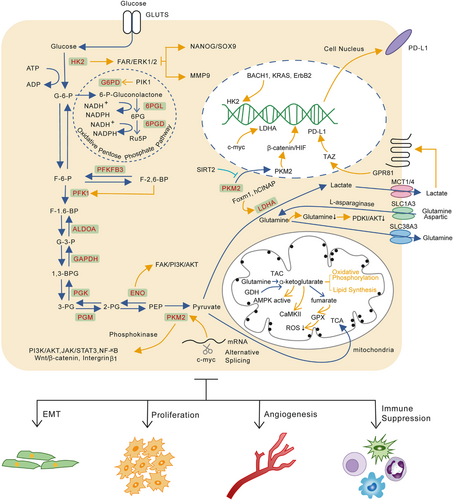
2 GLUCOSE METABOLISM AND CANCER METASTASIS
Generally, the predominant metabolic mode of glucose in normal cells is the oxidative phosphorylation pathway (OXPHOS) in an oxygen-rich environment and glycolysis when oxygen is insufficient. However, in the 1920s, the German scientist Otto Heinrich Warburg observed hepatocellular carcinoma (HCC) cells tended to uptake lots of glucose for glycolysis and produced metabolites such as lactic acid (aerobic glycolysis), named the “Warburg effect.”12 Subsequent studies revealed that highly malignant or metastatic tumor cells have stronger glycolytic ability than those with low metastatic capacity, suggesting a potential connection between abnormal metabolism, invasion, and metastasis characteristics. Accumulating evidence shows abnormal glucose metabolism caused by microenvironmental stimulation and dysregulation of genes greatly affects cancer metastasis.
2.1 Glycolysis
2.1.1 Hexokinase
As the first rate-limiting enzyme in aerobic glycolysis, hexokinases (HKs) are responsible for converting glucose into glucose-6-phosphate (G-6-P). HKs have four isozymes (HK1, HK2, HK3, and HK4) in mammals. Among all of them, HK1 is the most widely distributed and predominant isoform in most tissues, while HK2 possesses the highest enzyme catalytic activity mainly expressed in insulin-sensitive tissues.13 However, increased expression of HK2 in a variety of tumors triggered widespread attention.14, 15 In animal experiments, HK2 is essential for oncogenesis in Kras-driven lung cancer and ErbB2-driven breast cancer.16 Interestingly, the expression level of HK2 is higher in the metastasis lesions compared with primary lesions in some tumors, suggesting important function of HK2 in the process of tumor metastasis.17
Tumor cells can take up a large amount of glucose through abnormal expression of HK2. Decreasing HK2 expression or inhibiting its activity can significantly reduce the glycolysis rates and suppress tumor growth, invasion, and metastasis both in vitro and in vivo.18, 19 In ovarian cancer, HK2 promotes metastasis potential and maintains cancer cells stemness separately via FAK/ERK1/2/MMP9 and FAK/ERK1/2/NANOG/SOX9 cascade.17 Meanwhile, many factors can regulate HK2 expression levels and activity at the transcriptional, translational, and posttranslational levels at different metastatic stages.20 In lung cancer, antioxidant stabilizes the transcription factor BTB and CNC homology 1 (BACH1), thereby activating transcription of HK2 and GAPDH and stimulating lung cancer metastasis by increasing glucose uptake, glycolysis rates, and lactate secretion.21 Protein kinase B (AKT2) can phosphorylate the T473 site of HK2 to upregulate NF-κB, HIF1α, MMP2, and MMP9 and increase the invasion, tumorigenesis, and metastasis of colon cancer cells in vitro and in vivo.22 A recent study shows that atonal bHLH transcription factor 8 (ATOH8) can actively respond to laminar shear stress (LSS) and transcriptionally activate the expression level of HK2, enhancing glycolysis of circulating colorectal cells, by which CTCs can survive in the circulation system and colonization in distant organs.23
2.1.2 Phosphofructokinase
There are two subtypes of phosphofructokinase (PFK), PFK-1 and PFK-2, which catalyze the same substrate, but results in different production.24 PFK1 is the second rate-limiting enzyme in glycolysis which irreversibly catalyzes fructose-6-phosphate (F-6-P) to fructose-1,6-bisphosphate (F-1,6-BP). Compared to other rate-limiting enzymes in glycolysis and allosteric regulation of various metabolites, PFK1 is critical in determining the rate of glycolytic flux due to relatively low enzyme activity.25 In a major advance in 1981, Vanschaftingen et al. found fructose 2,6-bisphosphate (F-2,6-BP) was a novel and most powerful allosteric activator of PFK1 whose concentrations are maintained by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB/PFK2).26, 27 PFKFB/PFK2, which has kinase and phosphatase activities, can reversibly and dynamically catalyze F-6-P to F-2,6-BP with four kinds of isoenzymes (PFKFB1, PFKFB2, PFKFB3, and PFKFB4). PFKFB are responsive to hypoxia conditions and PFKFB3 has the most vigorous kinase-to-phosphatase activity and is widely overexpressed in many tumors, indicating its important role in oncogenesis and progression.28
PFKFB3 is highly expressed in gastric cancer and is associated with lymph node metastasis and the TNM stage.29 In breast cancer, an elevated level of PFKFB3 can increase VEGF-A expression and facilitate angiogenesis and cancer metastasis.30 Flynn et al. investigated the relationship between PFKFB3 and cancer cell dormancy. They discovered dormant cells highly expressed autophagic gen and downregulated PFKFB3 expression. Disrupting autophagy, caused by unbridled PFKFB3 expression, can reactivate proliferative programs of breast cancer stem cells (CSCs) and lead to recurrence of metastatic lesions.31 Given the critical role of PFKFB3 in tumorigenesis, tumor angiogenesis, and metastasis, it is a promising target for cancer therapy.
2.1.3 Pyruvate kinase
Pyruvate kinase (PK) catalyzes the last and physiologically irreversible step in glycolysis by converting phosphoenolpyruvate (PEP) to pyruvate and produce ATP. There are four PK isoforms (PKL, PKR, PKM1, PKM2) encoded by PKLR and PKM genes in mammals. PKM2 rather than PKM1 is widely expressed in actively proliferating cells like cancer cells, embryonic cells, and stem cells. The high expression level of PKM2 in cancer cells always indicated poor prognosis.32 Further investigation find that alternative splicing mediated by c-Myc gene is the main reason for tumor-specific expression of PKM2.33 PKM2 mainly exists in the form of dimers in tumors. On the one hand, it can shift cellular metabolism to the aerobic glycolysis model and promote lactate biosynthesis. On the other hand, the dimer form can character as phosphokinase to participate in signal transduction.34
Elevated PKM2 expression can promote carcinogenesis, migration, and metastasis in colorectal cancer (CRC),35 gastric cancer,36 liver cancer,37 renal cancer,38 breast cancer,39 and pancreatic ductal adenocarcinoma (PDAC)40 by increasing glucose uptake, lactate production, or its protein kinase activity, indicating PKM2 is a promising therapeutic target. Meanwhile, increasing evidence has shown the posttranslational modification (PTM), including phosphorylation, methylation, acetylation, oxidation, hydroxylation, glycosylation, and succinylation can also significantly affect the catalytic activity of PKM2 and cancer progression. In breast cancer cells, coactivator associated arginine methyltransferase 1 (CRAMT) can methylate PKM2 on arginine 445, 447, and 455 to stabilize tetramer formation of PKM2 and shifts the balance of metabolism from OXPHOS to aerobic glycolysis. Inhibition of PKM2 methylation significantly decreases cancer cells’ proliferation, migration, and metastasis.41 Xu et al. demonstrated that heat shock protein 90 (HSP90) phosphorylated PKM2 at L328 mediated by protein kinase glycogen synthase kinase-3β (GSK-3β), which promoted the glycolysis and malignant proliferation in liver cancer.42 However, previous investigations mainly focused on the relationship between PTM and tumor proliferation. Some other modification residues, whose physiological roles involve metabolic and nonmetabolic processes, remain to be further explored.
Additionally, PKM2 level in the circulation system has been regarded as a diagnostic marker in various cancers.43 Extracellular PKM2 promotes colon cancer migration by activating PI3K/Akt and Wnt/β-catenin pathway.44 Wang C et al. demonstrated that secreted PKM2 facilitated lung cancer metastasis via the Integrin Beta1/FAK signaling pathway.45
2.1.4 Other metabolic enzymes
Many nonrate-limiting enzymes reversibly catalyze other metabolic steps in the glycolysis process. Increasing studies have found these enzymes also significantly affect tumor glycolysis process and metastasis. For example, in recombinant mothers against decapentaplegic homolog 4 (SMAD4)-negative PDAC, phosphoglycerate kinase (PGK1) is upregulated and drives cell metastasis and proliferation via mitochondrial OXPHOS induction and glycolysis enhancement.46 Bu P et al. show that CRC cells undergo metabolic reprogramming via GATA6/ aldolase B (ALDOB) pathway after metastasizing to the liver. In this way, metastatic tumor cells increase fructose metabolism and provide fuel for central carbon metabolism to support proliferation in distant organs.47 However, another research shows low expression of ALDOB is related to poor prognosis and high recurrence and metastasis risk in HCC.48 The previous study of our group demonstrates that Insulin-like growth factor-1 (IGF-1) phosphorylates histone deacetylase 3 (HDAC3) on S424 via PI3/AKT/mTOR pathway, which results in deacetylation of enolase (ENO) on K394. The deacetylation of ENO2 obviously enhances glycolysis and metastasis of PDAC.49 In nonsmall cell lung cancer (NSCLC), upregulated ENO1 activates FAK/PI3K/AKT pathway and its downstream signals to manipulate the glycolysis, cell cycle, and promote epithelial–mesenchymal transition (EMT), a key metastasis step for tumor of epithelial origin.50
2.2 Lactate production and cancer metastasis
As the final export of glucose metabolism in the Warburg effect, lactate has been regarded as a useless metabolite for a long time. However, with the continuously in-depth research in TME and immunosuppression, the great contributions of lactate to immune evasion, tumor metastasis, and drug resistance have attracted more attention.51, 52
In general, when aerobic glycolysis in tumor cells comes to the pyruvate catalyzed by PKM, the pyruvate will mainly be catalyzed by lactate dehydrogenase A (LDH-A) to produce lactate. Expression of LDH is closely related to the invasion and metastasis of liver cancer, cervical cancer, head and neck cancer and CRC.53 Previous studies show the unusual PTM of oncogene c-Myc and LDH-A contributes to abnormal activation of LDH-A in tumors.54, 55 Downregulation of LDH-A significantly reverses the Warburg effect of tumors and inhibits tumor invasion and metastasis.56 As the catalytic production of LDH-A, lactate has been confirmed to regulate tumor invasion and metastasis. Several clinical studies reveal that the lactate concentration in tumors is also positively correlated with cancer metastasis.57 In human lung cancer cells, the G-protein-coupled receptor (GPR81) can respond and be activated by lactate derived from tumor cells. The activation of GPR81 increased the PD-L1 transcription and expression by activating the transcriptional coactivator TAZ, thereby leading to immune evasion of cancer cells.58
The latest research shows that lactate may be involved in the interaction between tumor cells and the cells in TME. The lactate secreted by tumor cells can promote transformation of tumor-associated macrophage (TAM) to M2 type, thereby facilitating tumor invasion and metastasis.59 Chen et al. found G protein-coupled receptor 132 (Gpr132), a membrane receptor on macrophages, can sense and respond to lactate in TME, resulting in function alteration of macrophage and distant metastasis of cancer cells.60 Cytotoxic T cells act as a critical player in antitumor immune response. Its capacity, at least in part, determines the progression of cancer cells. However, high lactate concentrations in TME can inhibit lactate export from T cells, which suppresses the proliferation and cytokine production of cytotoxic T lymphocytes up to 95% and leads to a 50% decrease in cytotoxic activity.61 In another research conducted by Gottfried revealed that lactate secreted by tumor cells is a vital element shaping the dendritic cell phenotype and may result in immune evasion of tumor cells.62 However, the molecular mechanisms involved in the impact of lactate on migration, immunosuppression, and angiogenesis need further investigation.
2.3 Pentose phosphate pathway and cancer metastasis
The pentose phosphate pathway (PPP), as the main pathway for anabolic substances, is closely related to tumor progression and metastasis. This pathway can be divided into the oxidative branch and the nonoxidative branch. Current research shows that the oxidative branch is closely related to tumor metastasis. Studies have revealed the effect of PPP on apoptosis and anoikis of cancer cells.63 McDonald et al. reported that apparent modification and reprogramming during the distant metastasis of PDAC rely on the oxidative branch of PPP to shunt. Inhibiting this pathway can selectively reverse reprogrammed chromatin, thereby inhibiting malignant gene expression and tumor metastasis.64 In addition, cell proliferation also depends on PPP. Ma et al. found that Polo-like kinase 1 (Plk1), a key regulator of cell mitosis, promoted cancer cell cycle progression and tumor metastasis by activating PPP.65 Our group's research also found the high expression of PPP rate-limiting enzyme glucose-6-phosphate dehydrogenase (glucose-6-phosphate dehydrogenase, G6PD) is significantly related to liver cancer metastasis and poor prognosis of patients, indicating G6PD is a potential therapy target in liver cancer.66
2.4 Mitochondrial dysfunction and cancer metastasis
Mitochondria are highly dynamic and undergo constant fusion and fission to maintain physiological functions.67 Dysfunctional mitochondria will be selectively eliminated by autophagosome; this process is called mitophagy.68 However, increasing evidence demonstrates that mitochondrial dysfunction greatly affects cancer metastasis. Chen et al. revealed that BRCA1 deficiency inhibited mitochondrial fission and mitophagy via blocking ataxia-telangiectasia mutated (ATM)/AMP/activated protein kinase (AMPK)/dynamin-related protein 1 (DRP1), which induced to NLRP3 inflammasome activation and cancer metastasis.69 Compared to primary tumor cells, metastatic tumor cells in breast cancer upregulate mitochondrial fission protein DRP1and downregulate mitochondrial fusion protein mitofusin-1 (MFN1). In this way, mitochondria became more fragmented and formed more lamellipodia, which facilitates cancer metastasis.70 Meanwhile, our findings revealed that downregulation of MFN1, which manipulates the conversion of cell aerobic glycolysis to OXPHOS, was closely related to metastasis and poor prognosis of HCC. Decreased expression of MFN1 destroyed mitochondrial dynamics and triggered EMT of liver cancer. Treatment with glycolytic inhibitor 2-deoxy-D-glucose (2-DG) significantly suppresses the effects induced by depletion of MFN1.71
The glucose metabolic reprogramming and part regulation mechanisms are described as Figure 1.
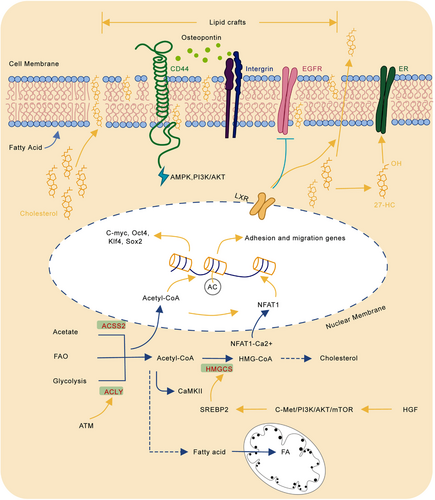
3 LIPID METABOLISM AND CANCER METASTASIS
Abnormal metabolism is one of the important characteristics of tumor cells. The metabolic changes of cancer cells mainly provide additional energy and metabolites for the proliferation and spread of cancer cells, among which lipid metabolism changes play a crucial role.7, 72 In general, the primary functions of lipids in cells are: 1) lipids are structural components of cell membranes and metabolic signaling molecules; 2) lipids store energy in lipid droplets (LD) and provide energy through β-oxidation to control redox homeostasis; 3) sterols are transcription factors that coordinate and regulate lipid synthesis.73-75 Experimental studies have clearly proven the crucial role of lipid metabolic reprogramming in tumor cell migration, invasion, and angiogenesis, indicating potential function in cancer metastasis and cancer therapy.
3.1 Changes in lipid metabolism of tumor cells
Cholesterol plays a vital role in the human body homeostasis. It is a basic component of cell membranes, especially plasma membranes. It is also a synthetic precursor of sterol hormones, bile acids, vitamins, etc. However, cholesterol provides suitable conditions for the occurrence and development of tumors. The specific molecular mechanism of cholesterol metabolism affecting tumor invasion and metastasis is still obscure. Recent studies have shown that changes in cholesterol metabolism can promote the metastasis and recurrence of tumor cells. On the one hand, cholesterol participates in forming lipid rafts. Lipid rafts are cholesterol- and sphingolipids-rich microdomains on the cell plasma membrane, which play an indispensable role in biological processes such as signal transduction and protein anchoring. Studies have shown that lipid rafts are closely related to signal transduction processes associated with tumor invasion and metastasis. For example, integrin and CD44 are important membrane proteins involved in osteopontin (OPN) activation of MAPK, PI3K/AKT, etc., promoting tumor invasion and metastasis. Interestingly, integrin and CD44 are located in lipid rafts, which makes activation of downstream signaling depend on lipid rafts stability.76 Previous research has shown that reducing plasma membrane cholesterol levels can destroy the lipid raft structure, thereby promoting CD44 shedding and inhibiting tumor cell migration.77 In addition, lipid rafts also significantly affect the epidermal growth factor receptor (EGFR) signaling pathway. EGFR pathway is an important signaling in tumor invasion and metastasis.78 Recent studies have found that activated liver X receptor (LXR) can increase cholesterol efflux in cancer cell. It can suppress the recruitment of sorafenib-dependent mesenchymal–epithelial transition factor (MET) and EGFR in lipid rafts. This experiment suggests LXR receptor agonists can be used as potential clinical sensitizers to improve the antitumor efficacy of sorafenib, illustrating the crucial role of cholesterol metabolism in the targeted therapy.79 On the other hand, cholesterol-related metabolites also significantly affect the biological behavior of tumors. Our previous work shows hepatocyte growth factor (HGF) from the liver environment activates the sterol regulatory element binding transcription factor 2 (SREBP2)-dependent cholesterol biosynthesis by activating the c-Met/PI3K/AKT/mTOR axis in CRC cells and thereby promoting CRC liver metastasis.80 In addition, the accumulation of cholesterol metabolite 27-hydroxycholesterol (27-HC) can increase the activation of estrogen receptor (ER) and promote the invasion and metastasis of breast cancer.81 Two important metabolites in cholesterol metabolism, farnesyl pyrophosphate (FPP) and geranyl geranyl pyrophosphate (GGPP) participate in the MAPK signaling pathway. Reducing these two metabolic intermediates can delay the growth, migration, and invasion of glioma cells.82
In summary, reducing cholesterol and its metabolites levels can be used as potential clinical tumor prevention strategy. However, as an essential nutrient for human body, comprehensively inhibits cholesterol and its metabolism will inevitably lead to sever side effects. Therefore, targeted suppression of the cholesterol metabolism in tumor cells has the potential for future clinical applications.
Fatty acids, a type of molecule composed of a terminal carboxyl group and a hydrocarbon chain, is a common metabolite produced in lipid metabolism. They are important components of lipid molecules such as triglycerides, phospholipids, and cholesterol lipids. Fatty acids are also involved in many biological processes of tumor cells. First, fatty acids are basic synthetic components of phospholipids, which are important structural molecules of cell membranes. The rapid proliferation of tumor cells requires large amounts of fatty acids. Compared with normal cells, tumors usually upregulate the de novo synthesis of fatty acids, making them more independent from the lipids provided by normal cells.83-85 Many enzymes involved in the de novo synthesis of fatty acids have been proposed as biomarkers for the prognosis of certain types of cancer.86, 87 For example, the expression and activity of key genes in the fatty acid metabolism pathway, ATP-citrate lyase (ACLY) and fatty acid synthase (FASN), have been increased in many tumors. They are closely related to poor prognosis. Downregulating the expression of these metabolic enzymes or using specific inhibitors to weaken the activity of metabolic enzymes can restrain tumor growth.88 Second, certain types of tumors, such as prostate cancer, mainly rely on fatty acid oxidative (FAO) as the primary source of energy instead of glucose uptake.89 Since tumor cells have higher levels of reactive oxygen species (ROS) than normal cells, this enables them to activate proliferation and transform from epithelial cells to mesenchymal cells to support the expansion and metastasis of cancer. FAO can not only provide energy when the glucose is limited but also better maintain redox balance by increasing the level of nicotinamide adenine dinucleotide phosphate (NADPH) in the cell.90 Therefore, FAO occupies a vital position in the proliferation and progression of cancer. In addition, FAO is also affected by the TME. For example, the preferential metastasis of ovarian cancer to the omentum rich in fat cells, which will be described later. Thirdly, fatty acid metabolism also plays a crucial role in tumor metastasis. The latest research has found that hydroxyacyl-CoA dehydrogenase (HADHA) is highly expressed in liver cancer tissues. HADHA promotes the formation of invasive pseudopodia by increasing the β-oxidation activity of fatty acids and increases cholesterol synthesis to enhance the fluidity of the pseudopodia, leading to EMT and metastasis of liver cancer. In liver cancer, inhibition of HADHA/ Acetyl Coenzyme A (acetyl-CoA)/HMG-CoA/cholesterol axis can impede pseudopodia formation, thereby reducing EMT and tumor metastasis. These studies show that fatty acid metabolism has great effects on the migration and invasion of tumor cells and can be used as a potential treatment target.91
Tumor metastasis puts forward higher requirements on tumor cells. That is, disseminated tumor cells (DTCs) need to adapt quickly to the new environment and proliferate fast to settle at the new metastasis sites.3, 92 The resources required for these DTCs to adapt and settle down can come from the metastasis site itself. For example, colon cancer cells absorb extracellular creatine phosphate in the liver microenvironment to produce adenosine triphosphate, which is used for the survival of colon cancer liver metastasis.93 Ovarian cancer preferentially metastasizes to the omentum rich in fat cells. The fat cells provide sufficient lipids to produce adenosine triphosphate (ATP) and NADPH to control the metabolic stress during metastasis.
3.2 Acetyl-CoA metabolism promotes cancer metastasis
Acetyl-CoA is a key metabolic intermediate produced by glycolysis, fatty acid β-oxidation, and branched-chain amino acid (BCAA).94 Acetyl-CoA determines the balance of cell catabolism and anabolism and connects the metabolism of many important metabolites.95 Intracellular acetyl-CoA level dynamically regulates histone acetylation, which controls genes expression and epigenetically adjusts protein functions.96, 97 Compared to normal cells, the synthesis of acetyl-CoA in tumor cells through uptake acetate from the microenvironment has become a vital pathway due to the increased aerobic glycolysis.98 Previous studies have found that the key metabolic enzymes involved in acetyl-CoA metabolism showed high expression or increased activity in tumor cells, such as acyl-CoA synthetase short chain family member 1/2 (ACSS1/ACSS2), ACLY, and acetyl-CoA carboxylase 1 (ACC1), indicating its promotion in oncogenesis and progression.99, 100
Meanwhile, Acetyl-CoA metabolism affects multiple steps of tumor invasion and metastasis. Mechanically, intracellular acetyl-CoA metabolic enzymes can affect the EMT process of tumor cells. In breast cancer, leptin and transforming growth factor beta 1 (TGF-β1) can phosphorylate ACC1 and inhibit its function through activating the TAK-AMPK pathway, thereby increasing nuclear and plasma acetyl-CoA concentration and promoting acetylation of transcription factor Smad2, which induces EMT and ultimately facilitates breast cancer metastasis and recurrence.101, 102 Lu et al. demonstrate acyl-CoA thioesterase 12 (ACOT12) regulates the cellular acetyl-CoA levels and H3 acetylation in HCC. Downregulation of ACOT12 facilitates HCC metastasis via epigenetically inducing TWIST2 expression and the promotion of EMT.103 In addition to EMT, acetyl-CoA metabolism is closely related to tumor cell adhesion and migration. Research conducted by Lee et al. revealed acetyl-CoA level is positively correlated with activation of integrin signaling pathway and abundance of H3K27 acetylation in migration- and adhesion-related gene promoter of glioma cells.104 Further investigation reveals that ACLY-dependent alteration of acetyl-CoA promotes calcium uptake, which in turn activates nuclear translocation of nuclear factor of activated T cells (NFAT), which increase the expression of genes related to cell adhesion and migration.105 As it is well known, calmodulin kinase II (CaMKII) is a multifunctional serine/threonine protein kinases family. However, in prostate cancer, researchers demonstrate that activated CaMKII level is significantly elevated in tumors with lymph node or bone metastasis based on clinical samples. At the same time, the activation of CaMKII depends on the acetyl-CoA concentration, which indicates acetyl-CoA may enhance prostate cancer metastatic capability.106 Expect EMT and cell adhesion and migration, acetyl-CoA plays an important role in maintaining the function of CSCs. CSCs are similar to pluripotent stem cells in normal tissues, which can self-renewal, clonal growth, proliferation, and homing. Previous study investigates embryonic stem cells are good at synthesizing acetyl-CoA and promoting histone acetylation to maintain stemness using glycolysis.107 A recent study shows that hypoxia can induce the activation of ATM kinase in triple-negative breast cancer (TNBC). Oxidation-activated ATM upregulates the activities of glucose transporter-1 (GLUT1), PKM, pyruvate dehydrogenase alpha (PDHA) and ACLY, increases the uptake of glucose by tumor cells and induces pyruvate flows to the synthesis of acetyl-CoA instead of the tricarboxylic acid cycle (TCA). As a result, increased acetyl-CoA promotes histone H4 acetylation and leads to enhanced stemness of breast cancer cells.108 The lipid metabolic reprogramming of cancer cells is described as Figure 2.
4 AMINO ACID METABOLISM AND CANCER METASTASIS
4.1 Metabolic reprogramming of amino acid in primary lesions
Amino acids are also important raw materials for cell anabolism. They participate in important processes such as oncogenesis and progression. Previous studies have revealed that there is abnormally active glutamine metabolism in some tumors. These tumor cells do not necessarily depend on glucose but exhibit glutamine-dependent growth. This phenomenon is “glutamine formation addiction.”109 Glutamine mainly produces α-ketoglutarate (α-KG) in the mitochondria to provide raw materials for OXPHOS and lipid synthesis through the TAC. Unlike the previous belief that Warburg metabolism is the predominant metabolism mode of tumors, Bradley IR 's research has put forward a subversive conclusion. In the MC38 mouse CRC model, tumor cells tend to uptake glutamine rather than glucose. And glutamine metabolism can significantly inhibit glucose metabolism and infiltration of immune cells.110 The glutamine uptake by tumor cells seems to provide another way for immune escape and cancer metastasis, because of its inhibitory effect on immune microenvironment. Another study suggests TNBC, with high metastasis potentiality and poor prognosis, dependents on extracellular glutamine for growth.111 Maybe “glutamine formation addiction” is one of the signs of tumor metastasis.
Therefore, in addition to the metabolism of glucose and fatty acids, amino acids are also important nutrients for tumor metastasis. In the process of tumor progression, oxidative stress caused by TME is an important factor in restraining tumor metastasis.112-114 For example, fumarate, an intermediate production of glutamine metabolism, can activate gluten. Glutathione peroxidase (GPx), a glutamine-related enzyme, is responsible for reducing the level of ROS inside tumor cells and maintaining the redox balance.115 ROS accumulation and redox imbalance may further promote tumor metastasis. In addition to the glutamine metabolism, other amino acids metabolism also plays a significant role in tumor metastasis. In patients with cancer disease, how nutrition remodeling (exogenous induction or self-reprogramming) changes their pathological processes, especially the metastasis process, is one of the current research hotspots. A recent study shows increasing dietary asparagine or upregulating the expression of asparagine synthetase (AS) promotes the progression of breast cancer metastasis via EMT. This study shows a potential mechanism for how the bioavailability of a single amino acid regulates the progression of metastasis.116 In a mouse tumor-bearing model, increasing leucine diet leads to transformation of tumor metabolism from glycolysis to OXPHOS, which has no effect on the primary tumor size but effectively reduces the size of metastatic foci.117 In breast cancer, Sun et al. demonstrated that solute carrier family 1 member 3 (SLC1A3) can promote cancer proliferation and metastasis by transporting aspartic acid and glutamate. In vivo, SLC1A3 weakens the antitumor efficacy of L-asparaginase (ASNase) by boosting the metabolism of aspartic acid, glutamate, and glutamine.118
4.2 The concentration of serum amino acid can predict cancer metastasis
As we all know, cancer is a systemic disease, and the metabolites in peripheral blood can sensitively reflect the progress of the tumor. Some scholars have found a significant difference in serum amino acid levels between CRC patients with lymph node metastasis (LNM) and patients with nonlymph node metastasis.119 In the melanoma study, scientists found that compared with the control group, the metabolism of patients with LNM or distant metastasis was significantly different. Among them, amino acid metabolism disturbances were the main ones, including arginine, proline, tryptophan, glutamine, glutamate, glutathione, and ornithine metabolism.120 Gastric cancer patients with LNM have significantly increased serum cysteine levels.121
4.3 EMT caused by amino acid metabolism
It is well-know that metabolic reprogramming of tumor cells at the primary site leads to the initiation of metastasis, which is a key step. A study of cell extracts and culture media based on MHCC97L and MHCC97H was used to reveal metabolic changes related to metastatic potential. Chen et al. found significant differences in amino acid metabolism between low metastatic MHCC97L and high metastatic MHCC97H.122 In breast cancer, kynurenine secreted by tumors can induce the death of CD8+ T cells, thereby improving the ability of breast cancer to metastasize.123 Glutamate dehydrogenase 1 (GDH1)-mediated glutamine catabolism reprogramming promotes tumor metastasis in lung cancers with klotho beta (KLB) deletion.124 Studies have pointed out that the upregulation of solute carrier family 38 member 3 (SLC38A3) can lead to the transport of glutamine, histidine and activation of PDK1/AKT signaling, thereby causing the EMT process of NSCLC and facilitating metastasis.125
4.4 Metabolic reprogramming of amino acid in metastasis lesions
After reaching the target organ, tumor cells also need a supply of nutrients. Many scholars have found that amino acid metabolism reprogramming can promote the colonization or proliferation of metastases. Proline dehydrogenase (PRODH) and proline catabolism increased in breast cancer metastases compared with the primary tumors of patients or mice. Meanwhile this amino acid metabolism pathway supports the growth of breast cancer cells in 3D culture.126 In addition, arginine is also a substrate of nitric oxide synthase (NOS). As an arginine transporter, solute carrier family 6 member 14 (SLC6A14) is upregulated in liver metastases and lymph node metastases of CRC.127
Lysine catabolism also produces glutamate, which modulates the redox state of CD110+ tumor-initiating cell to promote colonization of colon cancer in liver metastases.128 Therefore, the metabolic changes of metastases make the microenvironment more suitable for colonization and proliferation of metastatic tumor cells. As the research shows, the upregulation of glutamate decarboxylase 1 (GAD1) in breast cancer brain metastases causes a shift to glutamine metabolism in the microenvironment metabolism of brain metastases, thereby promoting the invasive properties of tumor cells.129
5 OTHER NUTRIENT METABOLISMS AND SIGNAL PATHWAY IN CANCER METASTASIS
5.1 mTOR signaling pathway
Metabolic reprogramming is usually regulated and mediated by a number of complex signaling pathways, among which mTOR signaling pathway is a vital one. mTOR is a highly conserved serine/threonine protein kinase from yeast to mammals, mainly involved in metabolism and growth control. It consists of two complexes, mTOR complex 1 (mTORC1) and mTOR complex 2(mTORC2). mTORC1 is sensitive to rapamycin while mTORC2 is not.130 Studies in many cancers have found that mTOR is usually activated by mutations in its upstream regulators, including mutations in PI3K3, PTEN, etc.131, 132 mTORC1 activation can be induced by nutrition and growth factors. Nutrients, particularly amino acids, promote translocation of mTORC1 from the cytoplasm to the lysosomal surface. According to current studies, among amino acids, leucine, arginine, and glutamine are effective activators of mTORC1. The activation of mTORC1 changes the metabolic process and the concentration of metabolites in tumor cells, and the change of the concentration of metabolites in turn regulates the activation of mTORC1.133 While compared with mTORC1, growth factor signaling alone is sufficient to activate mTORC2. But the process needs to be further studied.
In terms of biological functions, mTOR promotes the synthesis of various nutrients such as proteins, nucleotides, fatty acids and lipids, and inhibits catabolic processes such as autophagy. mTORC1 and mTORC2 regulate cell growth and metabolism mainly through phosphorylation of key metabolic enzymes or through downstream signaling effects.134 Interestingly, it has been found that there are differences in the activation of MTOR signaling pathways between immune cells and tumor cells in the tumor microenvironment. In a mouse sarcoma model, tumor cells acquire more glucose in the tumor microenvironment. MTOR signaling continues to be activated in tumor cells, glycolysis increases, and tumor progression occurs. On the contrary, decreased mTOR signaling and glycolysis in T cells affect T-cell function and cause immunosuppression.135
5.2 AMPK signaling pathway
AMP-activated protein kinase (AMPK) is a crucial cellular energy sensor.136 Although AMPK is known as a negative regulatory pathway for tumor proliferation, it often has multiple roles, such as causing tumor invasion and metastasis. It involves a variety of metabolic pathways via regulation of a series of biological processes, including glucose metabolism, lipid biogenesis, and protein synthesis,136 and leads to the occurrence and development of tumor metastasis through metabolic reprogramming. As we all know, LKB1/AMPK signaling downregulates SNAIL and ZEB1, which are the EMT marker proteins, and inhibits the invasion and migration of tumor cells, by regulating signaling pathways, such as those involving NF-κB, AKT, FOXO3, TGF-β, and mTOR.137 Low AMPK-induced metabolism can promote EMT and increase the risk for lung cancer metastasis.138 Chen et al.139 also found that the lack of AMPK activation promotes the invasion and metastasis of pancreatic cancer.
Downregulation of AMPKα1 expression in advanced breast cancer and poor clinical results, ablation of AMPKα1 expression, or inhibition of AMPK kinase activity leads to disruption of E-cadherin-mediated cell-cell adhesion in vitro and increased breast cancer metastasis in vivo.140 Fatty acid synthase (FASN) regulated AMP-activated protein kinase (AMPK)/mechanistic target of rapamycin (mTOR) pathway in CRC cells, then FASN enhanced CRC cell proliferation and metastasis.141 Upregulation of CXCL17 expression was observed in HCC, which is correlated with poorer histological stages and outcomes, chemokine CXCL17 reinforces malignant invasion and metastasis via the LKB1-AMPK pathway.142
Scholars found in the mouse metastasis model that AMPK-mediated phosphorylation of PDHA drives PDHc activation and TCA cycle, so that cancer cells can adapt to the metastatic microenvironment.143 High expression of AMPK signaling pathway in tumor patients often indicates a worse prognosis, such as breast cancer.144 All in all, after the AMPK signal pathway is inhibited by certain factors, it can promote the development and metastasis of tumors. With the deepening of research, these factors and the AMPK signal pathway may become relevant targets for tumor treatment.
5.3 Redox metabolism and cancer metastasis
Redox metabolism is a subcategory of cell metabolism, in which the central metabolite is ROS. High levels of ROS induce cell death through destroying cellular molecular and structure including proteins, nucleic acids, etc., while low levels of ROS make contribute to promote tumor growth, invasion, and metastasis. As mentioned above, redox homeostasis and ROS is closely related to glucose, lipid, and amino metabolism. Many previous reviews had comprehensively introduced the function of ROS and redox metabolism in oncogenesis and metastasis.145-147
To some extent, ROS is a promotor for cancer metastasis. In 2008, Kaori et al. revealed ROS could cause mitochondrial DNA (mtDNA) mutations, which led to the deficiency in respiratory complex I activity and tumor cell metastasis.148 In HCC, serine hydroxymethyltransferases cytoplasmic isozyme (SHMT1) represses NADPH oxidase 1 (NOX1)-mediated ROS production, and then inhibits the metastasis of HCC. In addition, ionizing radiation (IR) caused by radiation therapy may induce EMT and subsequently lead to cancer invasion and metastasis.149 ROS is proven to mediated IR-induced cancer progression via activating oncogenic pathway like NF-κB,150 MAPK,151 etc.
However, from other aspect, accumulation of reactive oxygen species (ROS) may prevent cancer metastasis due to an inefficient antioxidant response in cancer cells.152 Due to the dual nature of ROS, there are huge obstacles in developing drugs targeting ROS.147 How to balance redox homeostasis and ROS production in cancer therapy needs further research.
5.4 Iron metabolism and cancer metastasis
Arising studies reveal emerging role of iron metabolism in cancer metastasis.153-155 Iron, an essential nutrient in cells, is a mixed blessing. On the one hand, iron is required to maintain physiological function and process for cells. Meanwhile, iron can produce free radicals by flexibly converting between Fe2+ and Fe3+ forms, by which mediate redox and electron transfer reactions156; On the other hand, intracellular iron pools are related to ROS and lipid peroxidation. Excessive iron or imbalance of iron metabolism would result in cell death and dysfunction due to toxicity of free iron.157 Chi et al. find, in leptomeningeal metastasis (LM) lesion, inflammatory cytokines secreted by macrophages can induce cancer cells to overexpress iron-binding protein lipocalin-2 (LCN2) and its receptor SCL22A17. In this way, metastatic cancer cells can up-intake limiting iron from TME and suppress macrophage iron-dependent immune activity.158 In HCC, the increase of iron concentration can improve metastatic potential of HepG2 cells.155
Ferroptosis is a new form of regulated cell death, which is characterized by the iron-dependent accumulation of lethal lipid peroxides and involved in many critical diseases.159 Recent studies have revealed cancer cells resistance to ferroptosis increased metastasis. Jessalyn et al. have found lymph can protect metastasizing melanoma cells from ferroptosis. Compared to blood composition, lymph fluid has higher levels of glutathione and oleic acid, and less free iron, which can protect cancer cells from decreased oxidative stress and increase their survival in lymph experience.160 In renal cell carcinoma, KLF2 can impair transcription of glutathione peroxidase 4 (GPX4), a central regulator of ferroptosis, inhibiting cancer cell migration and invasion.161, 162 In conclusion, in addition to glucose and lipid metabolism, other metabolites may also participate in the cancer progression. More comprehensive information and analysis of metabolites in cancer metastasis is necessary.
6 METABOLIC REPROGRAMMING IN TME
6.1 TME
In addition to the above-mentioned genetic changes that mediate cell-autonomous metabolic reprogramming, the progression and spread of cancer also depend on nutrition and oxygen in the TME. TME is a complex system consisting of tumor cells, cancer-associated fibroblasts (CAFs), endothelial cells, innate immunocyte (macrophage, neutrophil, dendritic cell, myeloid-derived suppressor cell, natural killer cell), adaptive immune cell (T lymphocytes and B lymphocytes), and noncellular components including ECM, chemokines, metabolites, vesicles. Due to the high metabolic activity of tumor cells and insufficient blood perfusion, the accumulation of acidic metabolites often results in acidity, hypoxia, and nutritional deficiency around the tumor. The tumor communicates with the surrounding microenvironment to support the proliferation and spread of cancer.72 Therefore, changes in glucose, lipid, and amino metabolism are not only manifested in the influence of tumor cells themselves but also reflected in the interaction of cells around the tumor (Figure 3).
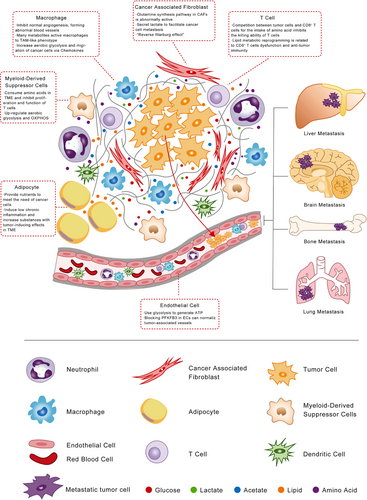
6.2 TAM
TAM is widely known as an important participant in angiogenesis by producing proangiogenic factors and cytokines (such as VEGF, PDGF, TGFβ) in a hypoxic environment.163 According to polarized phenotypes, TAM can be divided into two types, M1 (classically activated macrophage) and M2 (alternatively activated macrophage).164 Different polarization phenotypes of TAM exhibit different patterns of glucose metabolism, TAM-M1 enhances glycolysis, while TAM-M2 exhibits enhanced OXPHOS.165
However, mounting researchers have noticed the metabolic reprogramming and crosstalk between TAM and tumor cells, which can effectively drive angiogenesis and cancer metastasis,166 even resulting in tumor cells’ resistance to antiangiogenic therapies.167 The hypoxia in TME will make TAM significantly upregulate the expression of regulated in development and DNA damage responses 1 (REDD1), the negative regulator of mTOR pathway. REDD1inhibits mTOR pathway and hinders the glycolysis process of TAM. It will cause abnormal blood vessels formation rather than normal angiogenesis, promoting tumor EMT and dissemination.163 At the same time, TAM can respond to part of metabolites secreted by tumor cells through membrane surface receptors including monocarboxylate transporter1/4 (MCT1/4)168 and T-cell death-associated gene 8 (TDAG8)169 to activate the downstream cascade reaction and promote TAM recruitment from circulation system, thereby, to facilitate cancer metastasis. For example, lactate derived from tumor cells can activate the Notch signal and increase the secretion of C-C motif chemokine ligand 5 (CCL5) in TAM. Secreted CCL5 increases the aerobic glycolysis and migration of breast cancer cells, induces EMT. Inhibiting aerobic glycolysis can significantly reduce breast cancer cells EMT.170 Another study showed TAM could promote progression and the Warburg effect of PDAC via CCL18/NF-κB/VCAM-1. However, increased VCAM-1 upregulates lactate production and secretion of lactate, which can activate macrophages to TAM-like phenotype, forming a positive feedback loop.171
A recent study has reported that M2 type TAM exhibits a high expression of glutamine synthetase. By inhibiting the activity of glutamine synthetase, M2 type can be transformed to M1 type, which in turn inhibits angiogenesis, immune escape, and ultimately inhibits tumor metastasis.172 Pieter G revealed that ovarian cancer cells promoted the cholesterol efflux of TAM and the consumption of lipid rafts, which increased the interleukin 4 (IL-4) signaling and inhibited interferon gamma (IFN-γ) signaling, thereby promoting tumor metastasis.173 Meanwhile, cancer cells release succinate into TAM and activate SUCNR1/PI3K/HIF-1α pathway, which activate macrophages to a TAM-like phenotype and facilitate cancer metastasis.174
6.3 T cell
T cell is one of the most important immune cells in the microenvironment with a double-sides effect on oncogenesis and cancer progression. On the one hand, activated CD8+T cells are equipped with strong cytotoxicity, which have powerful antitumor effects. On the other hand, activated CD4+ T cells can promote or impede tumor growth depended on different subtypes. For example, CD4+ Th1 cells can activate the antitumor function of macrophages and NK cells via secreting IFN- γ, while CD4+ Th2 cells and regulatory T cells (Treg) may suppress the immune response to tumor cells.175 Activated T cells’ energy supply mode changes from FAO and OXPHOS to glycolysis and glutamine metabolism.176
Recent studies showed a better understanding of cholesterol metabolism in T cells and cancer metastasis. Inhibiting acetyl-CoA acetyltransferase 1 (ACAT1), a critical gene of cholesterol metabolism, promotes the proliferation of CD8+ T cell and increases the cholesterol level in plasma membrane, leading to the enhancement of T-cell receptor cluster and signal transduction, and the formation of immune synapses. It increases the antitumor ability of CD8+ T cells.177 Similarly, the accumulation of cholesterol in TAM can increase the expression of CD36 in CD8+ T cells, thereby increasing fatty acids intake, causing lipid peroxidation and ferroptosis, leading to CD8+ T-cell dysfunction and antitumor immunity.178
Meanwhile, as mentioned above, tumors have “glutamine formation addiction.” What role does glutamine play in the tumor immune microenvironment aroused widespread concern? Solute carrier family 5 member 1(SLC1A5), as a transporter of glutamine, has been confirmed to be upregulated during T-cell activation and mediates the uptake of large neutral amino acid (LNAA) in activated T cells.179 In the case of glutamine deprivation, it leads to the differentiation of naïve CD4+ T cells into forkhead box P3-positive [Foxp3 (+)] Treg cells.180 Another study also showed that after restricting glutamine intake, CD4+ T cells highly expressed Foxp3.181 Many amino acid metabolisms also regulate Treg cells, such as tryptophan catabolism182 and its metabolite kynurenine.183 The intake of essential amino acids also has a significant promotion effect on tumor metastasis. After restricting intake, it can inhibit tumor growth and metastasis.184 Tumor cells compete with CD8+ T cells for the intake of methionine, thereby inhibiting the killing ability of T cells and impairing T-cell immunity. But methionine supplementation increases H3K79ME2, which improves expression of signal transducer and activator of transcription 5 (Stat5) in T cells.185 Meanwhile, methionine and RagD potentiate mTORC1 activation in CD8 T cells, thereby promoting the tumor immunity of T cells.186 The decomposition of tryptophan by tumor cells and antigen presenting cells expressing indolylamine 2, 3-dioxygenase leads to tryptophan deficiency in the TME and inhibits T-cell function and survival.187 High concentrations of L-arginine in the microenvironment can make T cells to switch from glycolysis to OXPHOS and promote the generation of central memorial-like cells, which plays an antitumor role in mouse tumor models.188
6.4 Neutrophils
Neutrophil is a member of innate immunocytes. However, in the past, we paid more attention to the effect of neutrophil immune function on metastasis, but seldom researched the promotion of neutrophil metabolic reprogramming on tumor metastasis. Lu et al. found that lung-infiltrating neutrophils are the source of nutrition for DTCs lung metastasis in a mouse model of breast cancer lung metastasis. As neutrophils infiltrate the lung interstitium, pulmonary mesenchymal cells induce lipid storage through soluble factors and cell–cell contact-dependent mechanisms. These lipid-rich neutrophils provide initial nutrition for metastatic tumor cells. After the metastatic cells enter the lung tissue, neutrophils transfer their stored lipids to metastatic tumor cells by releasing vesicles. At the same time, lipid-containing neutrophils in the body also promote lung metastasis of breast cancer. Neutrophils are used as energy source fuel to provide nutrition for metastatic tumor cells in the premetastasis preparation stage and lung tissue metastasis process, thereby promoting lung metastasis of breast cancer in mice. This is quite different from our previous view that immune cells inhibit tumor metastasis and adds a new layer of complexity to the function of immune cells in tumor metastasis. It is helpful to further explore the specific mechanism of immune cells in the tumorigenesis and development process, thereby providing a new idea for the clinical treatment of solid tumor metastasis.189
6.5 Myeloid-derived suppressor cells (MDSCs)
MDSCs are another type of immunosuppressives cell in TME, derived from bone marrow progenitor cells and immature myeloid cells. Under normal circumstances, it can differentiate into dendritic cells, macrophages, and granulocytes. Compared with peripheral MDSCs, tumor-associated MDSCs are upregulated in both anaerobic glycolysis and OXPHOS.190 Amino acid metabolism of MDSCs has great effects on inhibiting T-cell function. The high expression of many amino acid metabolism-related enzymes in MDSCs can greatly uptake and consume arginine, lysine, and tryptophan. Lacking these amino acids in TME will inhibit the proliferation and function of T cells.191-193 MDSCs and TAM194 express Arg1, resulting in the depletion of arginine in TME and ultimately leading to tumor immunosuppression by inhibiting T-cell function.195 In another way, Tumor-derived TGF-β triggers the TGF-β/mTOR/HIF-1 signaling pathway to activate HIF-1α and induces the expression of CD39/CD73 on MDSCs, which leads to adenosine accumulation and results in immunosuppressive.196
6.6 Endothelial cells (ECs)
Tumor vessels are abnormal in shape, size, tortuous, and morphologically heterogeneous, causing insufficient perfusion and accumulation of metabolites in TME. It subsequently causes a hypoxic and acidic environment, which facilitates the intravasation and dissemination of cancer cells. Meanwhile, vascular endothelial cells with abnormal structure and function cannot serve as an effective barrier to prevent tumor cells from invading the circulatory system to reach distant organs.197 Recently, ECs metabolism has gained attention as a therapeutic target for inhibiting angiogenesis.198, 199 Mounting studies showed that ECs primarily use glycolysis rather than OXPHOS to generate ATP. Cantelmon et al. found that ECs were highly glycolytic and blocking PFKFB3 reduced cancer cell invasion and intravascular metastasis Inhibition of PFKFB3 could improve the chemotherapy effect of primary and metastatic tumors.197
6.7 CAFs
CAFs, as the most important and abundant group of cells in the TME, are a subtype of fibroblasts with complex origin characteristics and functions. Mounting work has revealed that CAFs can affect cancer initiation and progression through cell–cell contact, metabolic reprogramming, secreting growth factors, and extracellular matrix proteins.200, 201
The glutamine synthesis pathway in CAFs is abnormally active. CAFs take in a large amount of glutamate in TME and synthesize glutamine through glutamine synthetase (GS) to meet the strong demand of tumor cells for glutamine, thereby maintaining tumor growth.202 Sun et al. investigated that hypoxic CAFs can generate lactate, which acts as a metabolic coupling between CAFs and breast cancer cells, facilitates breast cancer cell invasion through activating the TGFβ1/p38 MAPK/MMP2/9 signaling axis and activating the mitochondrial capacity in cancer cells.203 In contrast to the “Warburg effect,” limited studies proposed the conception of the “Reverse Warburg effect,” which indicated CAFs might suffer from metabolic reprogramming, such as mitochondrial dysfunction, hydrogen peroxide production, and aerobic glycolysis, under the stimulation of oxidative stress.204 In this way, CAFs could secrete L-lactate to adjacent cancer cells to promote oxidative mitochondrial metabolism. Mechanically, increased expression of GLUT1 and monocarboxylate transporter-4 (MCT-4) in CAFs can facilitate the export of glucose and lactate from CAFs to cancer cells and promote tumor growth and metastasis.205, 206 However, compared with the “Warburg effect,” supported by a large amount of evidence, “reverse Warburg effect” requires more research and evidence.
6.8 Adipocyte
As we all know, obesity is a risk factor for many chronic diseases, and the mortality rate of obese people among cancer patients is generally higher than patients with normal body fat.207-210 The ectopic accumulation of adipocytes in the internal organs may cause changes in the cell composition around the TME, thereby promoting the proliferation of the primary tumor cells. For example, the nutrients needed for the rapid expansion of ovarian cancer tumors are provided by the abnormal accumulation of fat cells in the TME.211 Moreover, excessive fat accumulation can induce low chronic inflammation and increase the levels of substances with tumor-inducing effects in the blood and TME, such as proinflammatory interleukins, vascular endothelial growth factor (VEGF), tumor necrosis factor alpha (TNFα), prostaglandin, etc. The increased inflammatory factors in TME appear to promote tumor angiogenesis, expansion, and distant metastasis.212, 213 The above-mentioned important metabolic link between adipocytes and tumorigenesis provides a direction for clinical prevention and improvement of cancer progression, that is, effective control of individual nutrition and metabolism levels, which need to be further studied and quantified.
7 POTENTIAL APPLICATIONS IN COMBATING CANCER METASTASIS
Given the critical impact of metabolic reprogramming on tumor metastasis, new medicines and drug combinations are gradually being researched and developed. Unlike traditional dietary interventions, the specificity and effectiveness of antimetabolites is because they can target key metabolic enzymes and important metabolites.
As mentioned above, aerobic glycolysis is a crucial energy resource for cancer cells, and many glycolysis-related enzymes are significantly upregulated, indicating that interfering glycolysis of cancer cells might be an effective strategy. 2-DG, silybin, and some small molecular inhibitors can limit the glucose uptake of cancer cells by interfering with GLUTs function.214, 215 Inhibitors targeting PFKFB3 targeting inhibitors, such as PFK15 and 3PO can obviously suppress cancer proliferation and metastasis through blocking glycolysis in variant cancers.198 However, glycolysis is also a common metabolic pathway of normal cells, Which means that nonselective or low-selective drugs can also cause significant damage to normal cells and result in severe side effects. Therefore, the development of selective antiglycolytic agents of tumor cells is a massive challenge for further research.
LXR agonists, including nonselective agonist T0901317 and selective agonist GW3965 and so on, have great effects on inhibiting cancer proliferation and metastasis.216 Recent research has revealed that T0901317 has antitumor effects by reducing the intratumoral abundance of Treg and inhibiting the expression of immunosuppressive genes in TAM.217 However, hyperlipidemia and neurotoxicity are the most common side effects of LXR agonists due to their low selectivity. Therefore, the development of more selective LXR agonists will benefit patients with malignment tumors. Meanwhile, FASN inhibitors, like small-molecular cyanobacteria, C75, Orlistat, and TVB-2640, can kill tumor cell through blocking cell membrane synthesis and other oncogenic signaling pathways. Among them, TVB-2640 has shown certain antitumor effects, and predictable and manageable safety profile in Phase I clinical trial (NCT02223247).218, 219 Meanwhile, Phase II about the single-center pharmacodynamic study of TVB-2640 in KRAS mutant NSCLC (NCT03808558) and HER2+ breast cancer (NCT03179904) is in progress.
In amino metabolism, GS activity is a mediator of M2-like macrophages' angiogenesis, immunosuppression, and metastasis promotion functions. Genetic or pharmacologic inhibition of GS has been verified to inhibit tumor progression and metastasis.220 Xiang et al. found that BPTEs, inhibitors of glutaminase, could inhibit the proliferation of tumor cells in vitro, and could inhibit the growth of tumors in a mouse liver cancer model and prolong the mouse's survival.221 Robert Leone et al. demonstrated that glutamine inhibitor JHU083 could effectively improve antitumor immunity after treatment without additional immunotherapy. In their study, JHU083 was a glutamine analogue that inhibited the enzyme based on glutamine,222 and CB-839 was another. It has also shown the significant antitumor effects in a mouse model of hematologic malignancies.223 In solid tumors, the TNBC subtypes showed the most incredible sensitivity to CB-839 treatment.224 Further research on the mechanism of metabolic reprogramming in tumors is needed, and it is believed that the concept of “metabolic immune checkpoint” will lead to the research and development of more metabolic targets and more metabolic therapies for tumors.
8 DISCUSSION
In conclusion, abnormal regulation of oncogenes and tumor suppressor genes are the main cause of metabolic reprogramming of cancer cells, which help them meet the demand for proliferation, invasion, and metastasis. Meanwhile, nontumor cells in TME also undergo metabolic reprogramming to promote or inhibit cancer metastasis. Targeting metabolic reprogramming is becoming an emerging prevention and treatment strategy for malignant tumors. However, molecular mechanisms of metabolic reprogramming in cancer cells and TME components are still undiscovered. The intracellular metabolic modes are dynamic, reversible, time-sensitive, and heterogeneous, which occasionally makes the results inaccurate by existing detection methods and brings challenges to preclinical research and clinical transformation.225, 226
Recently, with the rise of novel technologies like single-cell sequence and spatial transcriptomics, more comprehensive and detailed information was revealed to help understand the physiological and pathological mechanisms and relationships in cancer diseases. As for metabolomics, mass spectrometry imaging (MSI) and metabolic flux analysis (MFA) have attracted more and more attention. MSI, a new type of molecular imaging technology, can directly detect lots of known or unknown metabolites’ content and spatial distribution information from samples with high sensitivity and subcellular spatial resolution.227 It is a powerful weapon for revealing the synthesis, distribution, accumulation, and regulation mechanism of metabolites. However, MSI can provide comprehensive and large-scale information about metabolites, but cannot explain where they come from and where they go. MFA use stable isotopes, like 13C, 15N, and 18O, to demonstrate dynamic activities in metabolic pathways. And the combination use of MFA and liquid chromatography tandem mass spectrometry (LC-MS/MS) can expand detected metabolite coverage.225, 228 Therapies targeting metabolic pathways are emerging in recent years. However, the regulation of metabolic pathways is extremely complicated due to variability and mutual transformation of metabolites. Nonselective or low-selective drugs can cause severe side effects to patients. So, some important questions need further studies to illustrate (1) the different metabolism status between primary and metastatic tumors, and whether the difference can affect the treatment effect of metabolic therapy, immunotherapy, targeted therapy, or chemotherapy; (2) the metabolic reprogramming of cancer cells in primary and metastatic lesion caused by interventional, surgical, radiation, immune, or targeted therapy; and (3) the metabolic heterogeneity within tumor tissues. As for clinical research, more selective drugs targeting metabolic pathway and biomarkers for screening patients should be developed.
ACKNOWLEDGMENTS
We thank the member of Cancer Metastasis Institution of Fudan university for comments and advice. This study was funded by the Key Program of National Natural Science Foundation of China (81930074) and the Major Program of National Natural Science Foundation of China (91959203).
AUTHOR CONTRIBUTIONS
LXQ and WWZ designed the outline and directed the writing of the paper. SZX, JJP, and JFX collected related papers, retrieved the related studies, drafted manuscript, and prepared the figures. All authors critically revised the manuscript.
CONFLICT OF INTEREST
No conflicts of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




