DNA damage response inhibition-based combination therapies in cancer treatment: Recent advances and future directions
Tianen Chen, Suparat Tongpeng, Ziyi Lu, and Win Topatana contributed equally to this work.
Abstract
DNA molecules are subject to various lesions that can be detrimental to the cells. DNA damage response (DDR) pathways encompass a variety of mechanisms that cells employ in response to DNA damage. While DDR promotes genomic stability in normal cells, it also protects cancer cells from DNA lesions, particularly against exogenous DNA-damaging agents. Therefore, DDR pathways can be exploited to account for resistance to chemotherapy and radiotherapy and have the potential to be targeted in cancer treatment. Apart from the poly (ADP-ribose) polymerase (PARP) inhibitors used in BRCA-mutant cancers, other DDR inhibitors are being developed and tested in clinical trials. Based on the synthetic lethality theory, combination therapies utilizing DDR inhibitors have been explored and are currently undergoing clinical trials, with promising results in cancer treatment. Combination therapies typically employ a DDR inhibitor to sensitize cancer cells to traditional chemotherapeutic agents, radiotherapy, immunotherapy, and even PARP inhibitors. Herein, we focus on recent advances in DDR inhibitor-based combination therapies other than PARP inhibitors, explore the advantages and disadvantages of the strategies, and discuss the current challenges and future perspectives.
1 INTRODUCTION
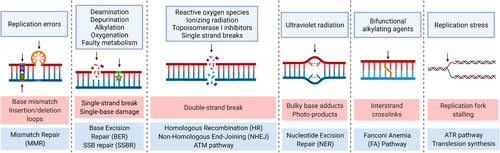
DNA is a double-stranded molecule that stores genetic information in all living organisms. It is a relatively stable molecule in comparison to single-stranded nucleic acids, owing to the hydrogen bonds between the opposite bases from the antiparallel strands, formed abiding by the complementary base pairing rule. This feature enables DNA to accurately record and replicate genetic information with few errors. Nevertheless, DNA is susceptible to various physical and chemical agents, resulting in a variety of DNA lesions.1 Endogenous or environmental DNA-damaging substances can cause damage spontaneously or on a deliberate basis. Spontaneous DNA alterations include dNTP misincorporation and undesired modification of the DNA bases such as deamination, depurination, alkylation, etc.2 Metabolic activities contribute to another significant endogenous source of DNA damage, reactive oxygen species (ROS), particularly in cancer cells with enhanced metabolic activities.3 In addition, environmental DNA-damaging agents play a significant role. Endogenous ROS and environmental ionizing radiation (IR), ultraviolet radiation, DNA-damaging chemical agents, etc., can induce DNA lesions via single base oxidation, intra- and interstrand covalent crosslinks, DNA adducts, and formation of DNA single- and double-strand breaks (SSBs and DSBs).2, 4 SSBs refer to discontinuities in one of the DNA strands caused mostly by ROS attack, base excision repair (BER), and defective enzyme activities, most notably the abortive activities of topoisomerase I.5 DSBs can result from the vicinity of two SSBs, one unrepaired SSB encountered by the DNA-replication apparatus, IR, or ROS.6, 7 Among all types of DNA damage, DSBs are extremely difficult to repair and the most detrimental. If DSBs are not repaired, they may induce gene mutations, chromosomal aberrations, carcinogenesis, and cell death.8 Other accumulating DNA lesions may also lead to increased genomic instability and vulnerability to exogenous DNA-damaging agents.
To address the hazards posed by DNA lesions, cells have evolved the DNA damage response (DDR), which is a defensive mechanism against diverse DNA lesions that can detect, signal, and repair the damage via a sequence of enzymatic reactions mediated by several proteins.6 Multiple DDR pathways can be activated in response to various types of DNA damage including mismatch repair (MMR), BER, SSB repair (SSBR), nucleotide excision repair (NER), translesion synthesis (TLS), Fanconi anemia (FA) pathway, homologous recombination (HR), canonical and alternative nonhomologous end-joining (NHEJ), etc.9 Numerous proteins are involved in sequential steps of each pathway including detecting, signaling, recruiting, and repairing. The functions of these proteins are being found and elucidated, as is their critical roles in repair pathways and cell survival. DDR is gaining increased interest as a result of its critical functions in cancer treatment. It is suggested that the majority of cancer cells contain defects in the DDR pathways, allowing for malignant growth and a greater likelihood of selection during tumor development.6 When one or more DDR pathways are impaired, cancer cells develop a higher dependency on alternative DDR pathways to maintain the stability of their chromosomes,10 which creates a potential target for synthetic lethality (SL) and makes it feasible to develop drugs targeting those specific DDR pathways in cancer treatment. Moreover, it is commonly assumed that highly effective DDR pathways contribute to the repair of extrinsic DNA damage in cancer cells, which helps to explain in part why cancer cells are resistant to DNA-damaging chemotherapy and radiotherapy. In terms of innate immune responses, membrane-bound DNA fragments derived from DDR inhibition or antitumor therapeutics can activate innate immune responses by inducing type I interferons and recruiting T-cells against cancer.11 Therefore, it is imperative to develop therapeutic methods that combine traditional antitumor agents with DDR inhibitors to minimize drug resistance, prevent undesired DNA repair, and maximize antitumor effects. Since the successful application of PARP inhibitors, an increasing number of DDR-inhibiting drugs are being studied and demonstrating promising results, particularly in combination therapies. This review will introduce SL concepts and DDR pathways. Furthermore, preclinical studies and clinical trials of DDR inhibitor-combined cancer treatment will be summarized. Finally, we will discuss the future prospects of DDR inhibition-based combination therapy.
2 DDR INHIBITION VIA PARP INHIBITORS
Clinically, the most successful DDR inhibitors to date are the poly (ADP-ribose) (PAR) polymerase (PARP) inhibitors for tumors with breast cancer gene (BRCA) BRCA1 or BRCA2 mutations. PARPs, a family of enzymes in response to DNA SSBs, can catalyze the transfer of ADP-ribose residues from NAD+ onto target substrates, building a PAR chain that serves as the DDR signaling and regulatory molecule in post-translational modification.12 PARP1, the best-studied and most important member of its family, recognizes and binds to the SSB through its DNA-binding domain, after which PARP1 undergoes a conformational change that activates the cleavage of NAD+ into ADP-ribose and nicotinamide and the covalent attachment of the ADP-ribose to the substrate proteins or the already attached ADP-ribose group to elongate the chain.13 The negatively charged PAR chains then recruit X-ray repair cross-complementing protein 1 (XRCC1), a critical scaffold protein for the recruitment of multiple DNA-repairing proteins, to the site of SSB. Subsequently, PAR is rapidly degraded by PAR glycohydrolase, restoring PARP1 and other PARylated proteins to their deribosylated state in preparation for more rounds of SSB detection and signaling.14 Apart from the functions in response to SSBs, PARP1 plays an important role in DSB repair as well. It is still unclear how PARP1 functions in HR, yet it is indispensable in alternative NHEJ,15 which will be explained in detail in the corresponding chapter.
In normal cells, SSBs are primarily repaired by the PARP-mediated SSB repair pathway, with few lesions remaining unrepaired and transformed into DSBs in the S phase. DSBs that occur from duplicated unrepaired SSBs are primarily repaired by the BRCA1-dependent HR pathway. However, in BRCA-mutated cells, the HR pathway does not function normally, and thus, DSBs persist and lead to apoptosis.16 On the one hand, PARP inhibitors block the repair pathway for SSBs and trap PARP1 on DNA, thus, inhibiting the recruitment of DNA repair effectors and destabilizing the replication forks.17 The repair pathway for SSBs fails and the replication forks collapse, turning SSBs into DSBs. The resulting DSBs, as mentioned above, are unable to be repaired in HR-deficient cells and, hence, become lethal. On the other hand, PARP inhibition may cause replication stress and accelerate replication fork elongation regardless of fidelity,18 which induces DDR. As a result, PARP inhibitors induce cell death in BRCA-deficient cells, constituting a canonical SL interaction that is widely used in clinical practice nowadays.17, 19
As the first PARP inhibitor and the first drug targeting DDR, olaparib (Lynparza) was approved for cancer treatment in 2014,20 indicating a great advancement of DDR inhibition in cancer treatment and a huge breakthrough in precision medicine. With the promotion of PARP inhibitors, many patients with BRCA mutations have developed PARP inhibitor resistance, which poses a major challenge. Ray Chaudhuri et al.21 indicated that stabilization of replication fork and protection of nascent DNA from degradation confers chemoresistance in BRCA-deficient cells. In addition, loss of 53BP1 is suggested to reverse the defect in HR-mediated DSB repair in BRCA-mutant cells, restore HR function, and reduce their sensitivity to PARP inhibitors.22 A recent report suggests that 53BP1 is in control of DNA end processing and DSB mobility, which explains the resistance.23 It is quite common for cancer cells to be heterogenous and develop drug resistance, which calls for rational drug selection and combination strategies to prevail. PARP inhibitor-based combination therapies have been under study and shown promising results in both preclinical and clinical studies24 including concurrent and sequential combinations with chemotherapies, other DDR pathway inhibitors, immune checkpoint inhibitors (ICIs), chemical BRCAness inducers, epigenetic therapies, etc.25 The roles of PARP and its inhibitors are well studied, as well as the SL mechanism and combination strategies. Taking PARP inhibitors as an example, this review will focus on DDR inhibitors other than PARP inhibitors and dig deeper into their mechanisms and combination strategies.
3 APPROACHES TO EXPLOIT DNA DAMAGE
3.1 Synthetic lethality in DDR inhibition
The mechanism of PARP inhibitors perfectly corresponds to the concept of SL. SL is a classical concept that ideally explains the interactions between a DDR inhibitor and its facilitator, thus, laying the foundation of DDR-based combination therapy. The SL concept was first raised by geneticists to describe the different consequences of gene defects. SL refers to the scenario where a single-gene defect is compatible with the cell viability, while the synthesis or combination of defects in both genes results in cell death.17, 26 These defects include mutations, overexpression, gene inhibition, etc., all of which can lead to abnormalities in gene expression.27 Notably, as epigenetic changes join Knudson's two-hit theory and are closely related to gene expression,28 silencing of DDR genes by aberrant epigenetic changes is a feasible approach to create gene defects and can apply to the gene pairing in SL. SL usually requires a pair of genes that are indispensable for cell viability and complementary to each other. The prime example is the BRCA-PARP gene pair that enables the induction of cancer cell death by PARP inhibitors in tumors with BRCA mutations.
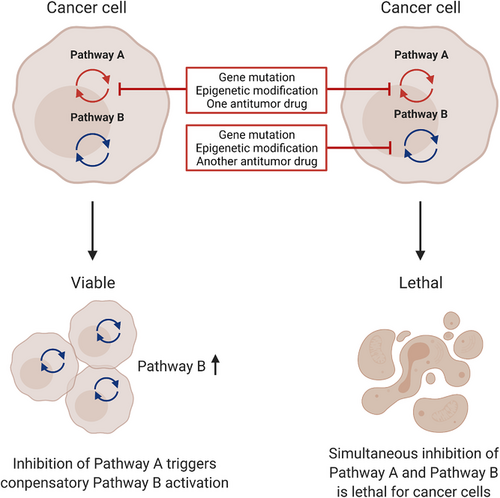
The simple mode of SL or nonconditional SL is not universal in every type of cancer. In addition to the central genes playing the key roles in the SL interactions, the tumor microenvironment (TME), heterogeneity of cancer cells, genetic background, as well as external factors can all influence the SL interactions.27, 29 In this case, the cancer cells retain part of viability after “synthetic toxic” partners exert their effects, instead of dying at once. Only under certain conditions, such as irradiation, administration of a DNA-damaging drug, suppression of specific genes, can the specific defects be lethal to the cells, and thus, the name, conditional SL. SL and conditional SL are common approaches in cancer treatment exploiting DDR inhibition, where some proteins involved in DNA repair have synthetic lethal interactions under certain conditions, especially gene mutations commonly seen in certain types of cancer. In clinical practice, SL and conditional SL are translated into DDR-based combination therapy, with one DDR inhibitor playing the central role and one or more facilitators enhancing the DDR inhibition effect. Modes of SL in cancer cells are illustrated in Figure 1. Some of the DDR inhibition-based combination strategies are listed in Table 2.
3.2 Replication stress and DNA damage
Accurate DNA replication and timely repair are pivotal biological processes to ensure genomic stability during cell proliferation. Unrepaired errors and damage in the course of DNA replication can accumulate and impede the replication process. Any hindrance to DNA replication that stalls, blocks, or terminates DNA polymerization, is called replication stress, characterized by slow-down of DNA replication and stalling of replication forks, and it is the primary cause of genomic instability, a hallmark of cancer.30, 31 To protect cells from this instability and ensure correct and prompt replication of DNA, several DDR pathways can be initiated and cell-cycle checkpoints will be activated.
With replication stress increasing, stretches of single-stranded DNA (ssDNA) bound with replication protein A (RPA) occur near the newly formed double-stranded DNA (dsDNA). The persistence of ssDNA generates a signal that activates the replication stress response and recruits a series of proteins, among which the central kinase is ataxia-telangiectasia and Rad3-related (ATR).32 The activation of the ATR pathway helps to stabilize and restart the stalled replication forks, and thus, relieve the replication stress.33 The ATR kinase is the primary regulator of replication stress, followed by checkpoint kinase 1 (CHK1) and WEE1, which are all targets for DDR inhibition and will be described in detail below. Other proteins in response to replication stress include BRCA1, BRCA2, carboxy-terminal binding protein-interacting protein (CtIP),34 etc., which protect replication fork from degradation.
Oncogenes can induce replication stress through inefficient origin licensing, rereplication, premature origin activation, impaired replication progression, or an accumulation of ROS.30 Moreover, cancer cells are prone to DNA damage and rely heavily on replication stress-response mechanisms to proliferate and withstand chemotherapy and radiotherapy. Many chemotherapeutic drugs target DNA replication and activate replication stress responses. For example, antimetabolites, such as 5-fluorouracil, compromise DNA and RNA metabolism to exert cytotoxicity; platinum-based chemotherapeutic drugs like oxaliplatin form platinum-DNA adducts to obstruct DNA replication; topoisomerase I inhibitors like irinotecan produce DSBs.35 These characteristics of cancer cells can be exploited in cancer treatment by increasing replication stress within cancer cells to induce cell death. One good example is the PARP inhibitors mentioned above, which not only prevent SSB repair but also stop and collapse replication forks to increase replication stress within cancer cells. More strategies targeting replication stress are being developed, mainly based on the inhibition of the ATR pathway.
3.3 DNA damage and immune responses
Alterations in the cancer genome lead to the expression of neoantigens that can subsequently present aberrant peptides bound to major histocompatibility complex (MHC) class I molecules on the surface of cancer cells. These distinguishing cancer-specific peptide-MHC class I complexes can be recognized by CD8+ T-cells that have been primed and activated by dendritic cells, resulting in activation of the innate immune responses against cancer cells.36 However, cancer cells can evade the immune responses through multiple approaches, most notably the inhibition of T-cell activation by cytotoxic T-lymphocyte antigen-4 and upregulation of immune response modulators like programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1). Agents that target these negative regulators have been developed and widely used clinically. To increase the antitumor effects, these ICIs are combined with DNA-damaging chemotherapy, ionizing radiation, and to our interest, DDR inhibitors, as the latter result in cell killing accompanied by the release of tumor antigens.37
In the cancer cells that are not killed by DNA-damaging agents or DDR inhibitors, the nuclear DNA molecules can be broken into DNA fragments, which are wrongly assembled into the nuclear envelope during mitosis and form micronuclei. Apart from DNA-damaging agents and DDR inhibitors, free DNA fragments can also be generated at stalled replication forks and as DNA repair intermediates.38 Under high levels of replication stress or defects in DDR pathways, these fragments accumulate and escape from their binding proteins. Harding et al.39 demonstrated that imperfect cell-cycle checkpoints allow passage through mitosis even without finishing the necessary repair pathway, leaving unrepaired DSBs and the formation of micronuclei. DNA fragments are transported to the cytoplasm in the micronuclei and recognized by cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS). cGAS is a DNA sensor that triggers innate immune responses through the production of the second messenger cyclic GMP-AMP (cGAMP), which binds and activates the stimulator of interferon genes protein (STING).40 STING then activates the downstream factors and finally elicits the expression of interferons and other cytokines of the innate immune system.41 Several clinical trials involving ICIs combined with DDR inhibitors are ongoing, including combinations with avelumab, durvalumab, and pembrolizumab, and are listed in Table 3.
4 TARGETING SSB AND DSB REPAIR
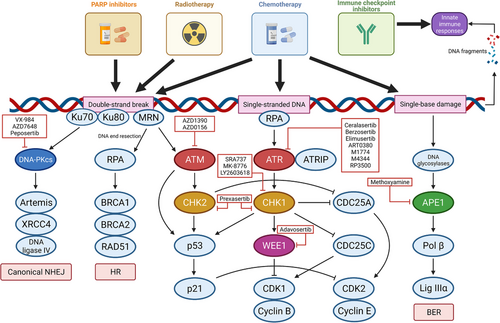
To deal with different types of DNA damage, there exist a variety of DDR pathways, and among them, multiple proteins (Figure 2). The proteins function as sensors of DNA damage, signaling proteins, and effectors participating in DNA repair. In this review, we focus mainly on the DDR pathways that have shown the potential to be exploited in cancer treatment. These principal DDR pathways and related proteins are listed in Table 1. Most of the proteins targeted in DDR inhibition are the ones playing the central roles in the corresponding pathways. The inhibition of the DDR pathways and synergistic mechanisms are illustrated in Figure 3. This section will discuss the DDR pathways, proteins, and corresponding inhibitors.
| DDR pathways | Predominantly related proteins | DNA lesions to repair | Cell-cycle checkpoint targets | References |
|---|---|---|---|---|
| Base excision repair (BER) | DNA glycosylases, APE1, XRCC1, FEN1, DNA polymerases, ligase I, ligase IIIa | Single-base lesion | [44] | |
| Single-strand break repair (SSBR) | PARP1, PARP2, XRCC1, FEN1, DNA polymerases, ligase I, ligase IIIa | Single-strand breaks (SSBs) | [49] | |
| Canonical nonhomologous end-joining | Ku70, Ku80, DNA-PKcs, XRCC4, | Double-strand breaks (DSBs) | G0/G1, intra G2 | [15] |
| Alternative nonhomologous end-joining | PARP1, polymerase θ | Intra-S | [15] | |
| Homologous recombination (HR) | RAD 51, ATM | Double-strand breaks (DSBs) | Intra-S, intra-G2 | [58] |
| DSB signaling pathway (ATM-mediated pathway) | ATM, MRN complex, CHK2, p53, p21 | Double-strand breaks (DSBs) | G1/S | [76] |
| Replication stress signaling pathway (ATR-mediated pathway) | ATR, ATRIP, 9-1-1 complex, CHK1, WEE1, CDC25 | Single-stranded DNA (ssDNA) | Intra-S, G2/M | [82] |
| Combination strategies | DDR inhibitor | Combined agent | Underlying mechanism | References |
|---|---|---|---|---|
| One DDR inhibitor with one DNA-damaging drug | APE1 inhibitor (Methoxyamine) | Temozolomide or Pemetrexed | Inhibition of DNA repair caused by chemotherapeutic drugs | [52] |
| ATR inhibitor (Berzosertib) | Topotecan, Carboplatin, Cisplatin, or Gemcitabine | [106] | ||
| CHK1 inhibitor (LY2603618) | Gemcitabine or Pemetrexed | [106] | ||
| WEE1 inhibitor (Adavosertib) | Cisplatin, Carboplatin, Gemcitabine or Paclitaxel | [126] | ||
| One DDR inhibitor with radiotherapy | DNA-PK inhibitor (Peposertib) | Radiotherapy | Inhibition of DNA repair caused by radiotherapy | [68] |
| ATM inhibitor (AZD1390) | Radiotherapy | [79] | ||
| ATR inhibitor (Berzosertib) | Radiotherapy | [106] | ||
| One DDR inhibitor with an ICI | DNA-PK inhibitor (Peposertib) | Anti-PD-L1 (Avelumab) | Increase in immune priming | N/A |
| ATR inhibitor (Ceralasertib) | Anti-PD-L1 (Durvalumab) | [94] | ||
| ATR inhibitor (Berzosertib) | Anti-PD-L1 (Avelumab) | N/A | ||
| Two DDR inhibitors | ATR inhibitor (Ceralasertib) | PARP inhibitor (Olaparib) (interchangeable) | Synergistic DDR inhibition | [87] |
| WEE1 inhibitor (Adavosertib) | PARP inhibitor (Olaparib) (interchangeable) | [143] | ||
| ATR inhibitor (Ceralasertib) | WEE1 inhibitor (Adavosertib) (interchangeable) | [144] | ||
| Combination of more than two agents | WEE1 inhibitor (Adavosertib) | Cisplatin and Radiotherapy | Combination of multiple effects | N/A |
4.1 Targeting SSB repair
4.1.1 Base excision repair pathway
BER is a highly conserved pathway in all living cells42 that corrects single-base damage and is mainly active in the G1 phase.43 Alkylations, oxidations, deaminations, depurinations, and SSBs, etc. can be repaired by the process.44 The initial step in the BER pathway is recognition and removal of single-base lesions by DNA glycosylases, classically monofunctional DNA glycosylases, which excise and remove the damaged deoxynucleoside base through hydrolysis of the N-glycosidic bond,45 leaving the strand with an abasic site. The resulting abasic site is recognized by an apurinic or apyrimidinic endonuclease that cleaves the site and leaves a sugar attached to the 5′ site of the nick. DNA polymerase β then removes the sugar attached to the 5′ phosphate. The gap is finally sealed by DNA ligase IIIα in complex with XRCC1, finishing the short patch repair.46 As BER primarily repairs single-base damage, DNA-damaging agents that exert their effects through single-base damage may have synergistic effects with BER pathway inhibitors. Trivedi et al.47 have found that BER contributes significantly to the repair of temozolomide-induced DNA damage.
4.1.2 Single-strand break repair pathway
The SSBR pathway is generally considered a specialized subpathway of BER,48 which can be divided into four basic steps, SSB detection, end processing, gap filling, and DNA ligation.49 SSBR starts with SSB recognition and signaling primarily by PARP1. PARP1 binds to the breaks and is thereby activated. It is suggested that PAR synthesis catalyzed by PARP1 promotes SSBR by recruiting XRCC1 and modifying chromatin structure.14 XRCC1 can recruit a variety of proteins including apurinic/apyrimidinic (AP) endonuclease 1 (APE1), polymerase β, polynucleotide kinase/phosphatase (PNKP), aprataxin (APTX), aprataxin-and-PNKP-like factor (APLF), and tyrosyl DNA phosphodiesterase 1, which form a protein complex containing the enzymes necessary for end processing. The following steps, gap filling, and DNA ligation, are finished in the same manner as the BER pathway. The main inhibitors of this pathway are the PARP inhibitors, as is discussed above.
4.1.3 Targeting APE1 in cancer treatment
Loss of the base in a nucleoside within a DNA strand is a common form of DNA damage, which leaves many AP sites, also known as abasic sites, in the DNA. Human AP endonuclease 1 (APE1) is the primary AP endonuclease that incises the DNA backbone hydrolytically and cleaves the phosphodiester bond at AP sites in BER.50 APE1 can also sense SSBs and act as an exonuclease to initiate 3′-5′ SSB end resection and DNA repair.51 Abbotts and Madhusudan52 suggest that APE1 expression has prognostic and predictive significance in cancer, as levels of APE1 correlate with resistance to chemotherapy and radiotherapy and that depletion of intracellular APE1 can sensitize cells to multiple DNA-damaging agents. Given the DNA-repairing function of APE1 and the correlation between APE1 expression and antineoplastic agent resistance, inhibition of the protein may sensitize cells to DNA-damaging agents, and thus enhance the antitumor activities of chemotherapy and radiotherapy.
TRC102 (methoxyamine, or in its hydrochloride form, methoxyamine hydrochloride) is a small organic amine that reacts with the aldehyde group in the sugar moiety at the AP site, through which it inhibits the lyase activity of AP endonucleases and blocks the BER pathway.53 In addition, TRC102-bound DNA acts synergistically with topoisomerase II as it prevents DNA religation after cleavage.54 As a BER inhibitor, TRC102 has shown promising results in clinical studies. In a phase I study (ClinicalTrials.gov Identifier: NCT00692159), 28 patients with advanced solid tumors received TRC102 in combination with pemetrexed, with the results showing that 15 of 25 patients evaluable for response achieved stable disease (SD) or better and that TRC102 was well-tolerated when combined with pemetrexed, with the maximum tolerated dose (MTD) per os at 60 mg/m2/d for 4 days.54 Another phase I clinical trial (ClinicalTrials.gov Identifier: NCT01658319) focusing on advanced hematologic malignancies showed that, among 20 patients diagnosed with advanced hematologic malignancies excluding acute myeloid leukemia, and administered with methoxyamine in combination with fludarabine, four patients achieved a partial remission, and eight achieved SD.53 In patients with relapsed solid tumors and lymphomas, TRC102 with temozolomide showed active results, with 4 of 51 patients experiencing a partial response (PR) and 13 having a best response of SD.55
APE1 activity is also associated with response to radiotherapy. Bobola et al.56 demonstrated that APE1 activity is predictive of outcome following radiotherapy in pediatric ependymoma. Another study using a nanoparticle-based small-interfering RNA delivery vehicle to knock down APE1 expression indicated that silencing of DNA repair sensitizes pediatric brain tumor cells to gamma irradiation.57
4.2 Targeting DSB repair
4.2.1 Homologous recombination pathway
HR is a common phenomenon in meiosis where it rearranges genes from parental chromosomes and produces new combinations of DNA sequences, whereas, in somatic cells, HR provides a method for DSB repair. Like alternative NHEJ, the broken DSB end is first resected by over 50 nucleotides into 3′ ssDNA tails, which is followed by assembling with the RAD51 filament and cofactors and searching for homology to form a displacement loop (D-loop). The D-loop extends and is mostly disrupted and subsequently repaired by the synthesis-dependent strand annealing (SDSA) pathway.58 Using a homologous chromosome as a template donor, one strand from the broken DNA invades the intact strand. SDSA disrupts the extended D-loop and anneals the newly synthesized DNA with the other broken end, resulting in noncrossover repair. RAD51 protein is an ATPase that plays a central role in HR as it forms right-handed helical filaments on ssDNA as nucleoprotein scaffolds to regulate the activities of interacting proteins.59 Single-strand annealing (SSA) is a branch of the HR pathway that requires a shorter resection of nucleotides. After end resection, the homologous repeat sequences flanking the DSB undergo RAD52-mediated annealing and the nonhomologous 3′ ssDNA tails are removed with deletion of the segment between the repeats.60 There are a large number of proteins involved in HR, including BRCA1 and BRCA2 in control of DSB resection and filament regulation, respectively.
4.2.2 Nonhomologous end-joining pathway
NHEJ is one of the primary pathways for DNA DSBs. In the canonical NHEJ pathway, the DSB is first recognized by the Ku70-Ku80 heterodimer, to which other proteins, including polymerases, nucleases, and ligases, can be recruited.15 Phosphatidylinositol 3-kinase-like kinase (PIKKs) are activated upon DSB formation and phosphorylate H2AX into γH2AX, a sensitive marker for DSBs.61 The nucleases degrade the DNA ends to generate small regions of microhomology between the strands to facilitate end joining. Multiple rounds of resection and addition may cause nucleotide deletion and addition after repair. DNA-dependent protein kinase (DNA-PK) plays a major role in canonical NHEJ and will be introduced below. Apart from the canonical NHEJ, alternative NHEJ plays another important role in DSB repair. Unlike canonical NHEJ, the DSB ends undergo a short-end resection in alternative NHEJ to generate 3′ ssDNA tails with microhomology. This pathway needs PARP1 as a sensor of DNA damage and polymerase θ to fill the gap.15 Alternative NHEJ functions independently of canonical NHEJ and competes for the repair of DSBs with other pathways.62
4.2.3 Targeting DNA-PK in cancer treatment
DNA-dependent protein kinase catalytic subunit (DNA-PKcs) is a serine/threonine protein kinase belonging to the PIKK family. DNA-PKcs, together with A-T mutated (ATM) and ATR, comprises the three PIKKs involved in DDR. It is an inactive kinase on its own and depends on a heterodimer that is formed by Ku70 and Ku80 proteins to direct it to the DNA and trigger its activity.63 Together with the Ku complex, DNA-PKcs then forms the activeDNA-PK, which is a serine/threonine protein kinase required for DSB repair by canonical NHEJ.64 DNA-PKcs is believed to have the primary function in DSB repair, not only in the canonical NHEJ pathway but also in multiple nodes of DDRs.65 Loss of DNA-PKcs function results in a marked decrease in the recruitment of additional DDR factors to DSBs,66 which explains the importance of DNA-PKcs in DSB repair and promises synergistic effects with other DSB repair modulators.
DNA-PKcs has altered expression and activity in certain types of cancer, whereas enhanced DNA-PKcs activity has been found to correlate with resistance to chemotherapy in chronic lymphocytic leukemia and poor outcomes.67 The DNA-PK inhibitor peposertib (also known as M3814) can sensitize multiple cancer cell lines to IR and DSB-inducing agents in vitro and in two xenograft models of human cancer in mice.68 In another preclinical study, M3814 synergistically sensitized p53 wild‑type acute myeloid leukemia cells to DSB‑inducing agents such as IR and anthracyclines via p53‑dependent apoptosis and enhanced the efficacy against leukemia cells in vitro and in an in vivo patient-derived mouse model when combined with the fixed‑ratio liposomal formulation of daunorubicin and cytarabine, CPX‑351 without increasing hematopoietic toxicity.69 A recent study (ClinicalTrials.gov Identifier: NCT02316197) of the DNA-PK inhibitor peposertib (formerly M3814) in patients with advanced solid tumors showed that peposertib demonstrated modest efficacy in unselected tumors and was well tolerated with the most common treatment-emergent adverse events (TEAEs) related to peposertib being nausea, vomiting, fatigue, and pyrexia.70 Other agents targeting DNA-PK include DNA-PKcs inhibitor VX-98471 and DNA-PK inhibitor AZD764872 which are still under development. Further clinical studies combining inhibition of DNA-PK and other treatments are ongoing.
5 ATM IS A MAJOR REGULATOR IN RESPONSE TO DSBS
ATM is a signaling kinase belonging to the PIKK family, which is mainly involved in DSB response. Mutation of the gene leads to ataxia-telangiectasia, an autosomal recessive genetic disorder characterized by unsteady gait and dilated blood vessels.73 ATM is recruited to DNA DSBs by the MRE11–RAD50–NBS1 (MRN) complex. CtIP associates with the MRN complex74 and stimulates MRE11 endonuclease activity to start DNA end resection. The MRN complex senses DSBs, binds to the broken site, and recruits ATM.75 ATM is then activated and transduces the DSB alarm to numerous downstream effectors, most importantly checkpoint kinase 2 (CHK2), to start the DSB repair process and activate cell-cycle checkpoints promptly.76 Besides, ATM can directly activate p53, which subsequently activates p21, a cyclin-dependent kinase inhibitor (CDK) belonging to the CDK serine/threonine kinase family, involved in cell cycle block, to pause the cell cycle at the G1 phase.
DNA DSBs are mainly repaired by NHEJ and HR pathways. ATM and DNA-PKcs respond to DSBs in the HR pathway and canonical NHEJ pathway, respectively, and are complementary to each other.77 Simultaneous inactivation of both ATM and DNA-PKcs leads to accumulation of DSBs and ultimately, cell death, forming a synthetic lethal interaction.78 Also, Lavin and Yeo79 found that targeting ATM sensitizes cells to DNA-damaging agents, which appears to be related to genetic instability due to impaired DSB repair. These findings make it potential to target ATM in combination with other anticancer treatments.
In a preclinical study, AZD0156, an ATM inhibitor, has been found to sensitize FaDu cells, a cell line of squamous cell carcinoma, to irradiation, and potentiates the effects of olaparib, a PARP inhibitor, on triple-negative breast cancer, gastric cancer, and non-small cell lung cancer cells.80 In another study, cotargeting of WEE1 and ATM by AZD1775 and AZD0156, respectively acts synergistically to not only reduce pancreatic cancer cell proliferation and migration, but also downregulates PD-L1 expression in vitro.81 A phase I clinical study (ClinicalTrials.gov Identifier: NCT02588105) is ongoing to assess the safety and primary efficacy of AZD0156 alone and in combination with olaparib or FOLFIRI. Recently, a first-in-human study (ClinicalTrials.gov Identifier: NCT04882917) of another ATM inhibitor, M4076, monotherapy has started to determine its pharmacological properties in participants with advanced solid tumors. More combination therapies need to be tested in clinical trials to enrich anticancer strategies based on ATM inhibition.
6 ATR-CHK1-WEE1 AXIS RESOLVES THE REPLICATION STRESS
6.1 Ataxia-telangiectasia and Rad3-related: Function and inhibition
ATR is a signaling kinase of the PIKK family, playing the major role as a sensor and regulator of replication stress. To address the replication stress, ATR maintains replication fork stability and promotes replication fork restart.82 The localization of ATR to the site of DNA damage is dependent on ATR-interacting protein (ATRIP) which, together with ATR, forms a complex recruited to RPA-covered ssDNA.83 However, this is still insufficient to fully activate ATR. An ATR activator, either topoisomerase II binding protein 1 (TopBP1) or Ewing tumor-associated antigen 1 (ETAA1), is necessary to directly activate ATR-ATRIP at sites of DNA damage.84 TopBP1 is recruited by the 9-1-1 complex (RAD9-RAD1-HUS1) loaded onto RPA-coated ssDNA-dsDNA junctions via the RAD17-replication factor C complex,84 while ETAA1 directly interacts with RPA and promotes replication fork progression and integrity.85 With ATR activation, the downstream kinases, most importantly CHK1 and WEE1, will be phosphorylated to pause the cell cycle at G2.
A preclinical study showed that the ATR inhibitor, VE-821, abrogated chemotherapy-induced cell cycle arrest, disrupted chemotherapy-induced CDC25A degradation, and sensitized ovarian cancer cells to a wide range of DNA-damaging agents, including cisplatin, topotecan, gemcitabine, and veliparib, as was observed in cells depleted of ATR by siRNA.86 In terms of synergistic effects with PARP inhibitors, ATR inhibitors have been found to work well. Acquisition of PARP inhibitor-resistance is accompanied by increased ATR-CHK1 activity in ovarian cancer models, which can be neutralized, in the same way as platinum resistance, by the combination of a PARP inhibitor and an ATR inhibitor.87 Cancer cells can develop resistance to PARP inhibitors mainly through the restoration of HR repair and replication fork protection,88 which is prevalent in clinical practice and has posed a tremendous challenge. Studies have found that combination therapy with cell-cycle checkpoint inhibitors, that is, inhibitors of ATR, CHK1, and WEE1, could enhance PARP inhibitors’ efficacy and circumvent the resistance.89 A study revealed that ATR inhibitors can disrupt BRCA1-independent RAD51 loading to DSBs and stalled forks in cells with PARP inhibitor-resistance and BRCA-1 deficiency, endowing ATR inhibitors with the ability to preferentially sensitize these cells to PARP inhibitors.90 Other studies have found that VE-821 can be a potent radiosensitizing agent for p53-negative HL-60 cells,91 and that VE-821 abrogate the intra-S checkpoint, inhibit the DNA elongation checkpoint induced by topoisomerase I inhibitors, and sensitize cancer cells to topoisomerase I inhibitors both in vitro and in mice bearing subcutaneous COLO205 tumors.92
Ceralasertib (also known as AZD6738) is a potent ATR inhibitor. Vendetti et al.93 revealed that when combined with conformal radiation, AZD6738 can potentiate CD8+ T-cell activity in the TME in a mouse model. Another mouse model-based study indicated that AZD6738 might have synergistic effects with radioimmunotherapy in controlling the proliferation of hepatocellular carcinoma.94 Nam et al. discovered that the combination treatment of ceralasertib with olaparib synergistically increased antitumor effect in vitro and in mouse models, and reduced PD-L1 expression to inhibit immune evasion in biliary tract cancer.95 A phase I study (ClinicalTrials.gov Identifier: NCT02630199) showed that ceralasertib combined with paclitaxel was well tolerated and effective in patients with refractory cancers, especially immunotherapy-resistant melanoma, with the most common TEAEs being anemia and neutropenia.96 The overall response rate was 22.6% in all patients and 33.3% in patients with anti-PD1 therapy-resistant melanoma. Another ATR inhibitor, berzosertib (also known as VX-970 or M6620), is well tolerated when administered with cisplatin in a phase I study (ClinicalTrials.gov Identifier: NCT02157792) and have a preliminary clinical effect in patients with advanced solid tumors, with 4 (15.4%) of the 26 eligible patients achieved PR and 15 (57.7%) achieved SD.97 The corresponding population pharmacokinetics analysis showed no evidence of a clinically significant pharmacokinetic interaction between berzosertib and evaluated chemocombinations, suggesting that dose modifications of berzosertib are not likely needed when administered in combination with various agents in patients with different advanced solid tumors.98 An early phase I study (ClinicalTrials.gov Identifier: NCT02487095) of ATR inhibitor M6620 has demonstrated that a combination of M6620 with topotecan is safe and active in platinum-refractory small-cell lung cancer that does not respond to topotecan alone.99 In this study, Thomas et al. reported 2 PR and 7 SD responses of the 21 enrolled patients, including 3 of 5 patients with platinum-refractory small-cell lung cancer showing PR or prolonged SD. Combining the phases I study and preclinical work, Thomas et al.100 discovered that small-cell lung cancer cells were vulnerable to multiple drugs targeting replication stress response, and that combination of M6620 and topotecan brought about remarkable clinical benefit with high response rates and durable responses in patients with platinum-resistant disease. In another phase I study of M6620, the ATR inhibitor was well tolerated in combination with carboplatin and proved appropriate to be given 24 h after carboplatin administration,101 namely delayed administration, based on the results of preclinical studies demonstrating that the maximum efficacy of ATR inhibition was achieved 12–24 h after DNA-damaging chemotherapy. A novel ATR inhibitor Elimusertib (also known as BAY1895344) is reported to exhibit strong synergistic antitumor activity combined with chemotherapy and monotherapy efficacy in cancer xenograft models with certain DDR deficiencies.102 A first-in-human trial in patients with solid tumors (ClinicalTrials.gov Identifier: NCT03188965) has demonstrated that oral treatment with BAY1895344 is well tolerated and shows antitumor activity in pretreated patients, especially those with ATM function loss.103 More ATR inhibitors are under development. Recently, a novel ATR inhibitor M4344 has been shown to synergize with a broad range of clinical anticancer drugs, including etoposide, gemcitabine, and cisplatin, using in vitro and in mouse xenograft models of multiple cancer cell lines, through inducing replication catastrophe, collapse of cell-cycle progression, and finally lethal DNA damage, while replication stress and neuroendocrine gene expression signatures can be predictive markers for M4344 therapy.104
The aforementioned studies corroborate the underlying mechanism of ATR inhibitors in blocking the cell-cycle checkpoints and DNA repair based on the SL concept. The results of the ATR inhibitor-based combination therapy so far have revealed its enormous potential in preclinical studies and clinical practice as well. The current clinical trials combining ATR inhibitors in cancer treatment are still ongoing and listed in Table 3. In light of the positive results from preclinical and clinical studies, as well as the numerous ongoing clinical trials of ATR inhibitors-based combination therapies, we hold the view that ATR inhibitors are the most promising DDR inhibitors and expect them to be approved in the clinical use next.
| DDR Target | Agent | Intervention | Disease | Phase | ClinicalTrials.gov Identifier and References |
|---|---|---|---|---|---|
| APE1 | Methoxyamine (TRC102) | Methoxyamine + Temozolomide | Adult Advanced Solid Tumors | I | NCT00892385 |
| Methoxyamine + Pemetrexed + Cisplatin + RT | Locally Advanced Lung Non-Squamous Non-Small Cell Carcinoma (Stage IIIA-IV) | I | NCT02535325 | ||
| Methoxyamine + Fludarabine | Relapsed or Refractory Hematologic Malignancies | I | NCT0165831953 | ||
| Methoxyamine + Cisplatin + Pemetrexed | Advanced Solid Tumors or Mesothelioma | I/II | NCT02535312 | ||
| TRC102 + Temozolomide | Relapsed Solid Tumors and Lymphomas | I/II | NCT01851369 | ||
| TRC102 + Pemetrexed | Advanced or Metastatic Solid Cancers | I | NCT0069215954 | ||
| DNA-PKcs | VX-984 (M9831) | VX-984 (M9831) + Pegylated Liposomal Doxorubicin | Advanced Solid Tumors | I | NCT02644278 |
| DNA-PK | AZD7648 | AZD7648 + Pegylated Liposomal Doxorubicin | Advanced Malignancies | I/II | NCT03907969 |
| Peposertib (M3814, Nedisertib) | M3814 + RT ± Cisplatin | Advanced Solid Tumors | I | NCT02516813 | |
| Peposertib + Capecitabine + RT | Locally Advanced Rectal Cancer | I/II | NCT03770689 | ||
| Peposertib + RT | Stage III-IV Local-Regionally Advanced HNSCC | I | NCT04533750 | ||
| M3814 + Avelumab ± RT | Advanced Solid Tumors | I | NCT03724890 | ||
| Peposertib (M3814) + Lutetium (Lu-177) Dotatate | Gastroenteropancreatic Neuroendocrine Tumors | I | NCT04750954 | ||
| Peposertib + Intensity-Modulated RT | Advanced HNSCC | I | NCT04533750 | ||
| Peposertib (M3814) + Hypofractionated RT | Locally Advanced Pancreatic Adenocarcinoma | I/II | NCT04172532 | ||
| Peposertib + Pegylated Liposomal Doxorubicin Hydrochloride | Ovarian and Related Cancers, High-Grade Serous and Low-Grade Serous Ovarian Cancers | I | NCT04092270 | ||
| Peposertib (M3814) + Avelumab + Hypofractionated RT | Advanced/Metastatic Solid Tumors and Hepatobiliary Malignancies | I/II | NCT04068194 | ||
| M3814 + Radium-233 dichloride + Avelumab | Advanced Metastatic CRPC | I/II | NCT04071236 | ||
| M3814 + MEC (Mitoxantrone + Etoposide + Cytarabine) | Relapsed or Refractory Acute Myeloid Leukemia | I | NCT03983824 | ||
| Nedisertib + RT + Temozolomide | Glioblastoma and Gliosarcoma | I | NCT04555577 | ||
| DNA-PK (and mTOR) | CC-115 | CC-115 | Advanced Solid Tumors and Hematologic Malignancies | I | NCT01353625145 |
| CC-115 + Enzalutamide | CRPC | I | NCT02833883 | ||
| DNA-PK and ATM | XRD-0394 | XRD-0394 + RT | Metastatic, Locally Advanced, or Recurrent Cancer | I | NCT05002140 |
| ATM | AZD1390 | AZD1390 + RT | Glioblastoma Multiforme and Brain Metastases from Solid Tumors | I | NCT03423628 |
| AZD1390 + RT | NSCLC | I | NCT04550104 | ||
| AZD0156 | AZD0156 + Olaparib/ FOLFIRI (Irinotecan + Fluorouracil + Folinic acid) | Advanced Solid Tumors | I | NCT02588105 | |
| ATR | Ceralasertib (AZD6738) | AZD6738 + Olaparib | Solid Tumor Malignancies | II | NCT03682289 |
| AZD6738 + Olaparib | Gynecological Cancers | II | NCT04065269 | ||
| AZD6738 + Paclitaxel | Refractory Cancers | I | NCT0263019996 | ||
| AZD6738 + Olaparib | Metastatic CRPC | II | NCT03787680 | ||
| Ceralasertib + Acalabrutinib | Relapsed or Refractory High-risk Chronic Lymphocytic Leukemia | I/II | NCT03328273 | ||
| AZD6738 + Acalabrutinib | Relapsed or Refractory Aggressive Non-Hodgkin's Lymphoma | I | NCT03527147 | ||
| AZD6738 + Gemcitabine | Advanced Solid Tumors | I | NCT03669601 | ||
| AZD6738 + Durvalumab | NSCLC | II | NCT02664935 | ||
| AZD6738 + Olaparib | Recurrent Ovarian Cancer | II | NCT03462342 | ||
| Ceralasertib + Olaparib | Recurrent Osteosarcoma | II | NCT04417062 | ||
| Ceralasertib + Durvalumab | Unresectable or Advanced Melanoma | II | NCT05061134 | ||
| Ceralasertib + Cisplatin/Carboplatin + Etoposide + Durvalumab | Extensive Stage SCLC in Treatment-Naïve Patients | II | NCT04699838 | ||
| Ceralasertib + Carboplatin/Olaparib/Durvalumab | Advanced Solid Malignancies | I | NCT02264678 | ||
| Ceralasertib + Trastuzumab Deruxtecan | Advanced Solid Tumors with HER2 Mutation | I | NCT04704661 | ||
| Ceralasertib + Olaparib | Isocitrate Dehydrogenase Mutant Solid Tumors | II | NCT03878095 | ||
| Ceralasertib + Olaparib | BRCA Germline-Mutated Advanced Breast Cancer | II | NCT04090567 | ||
| AZD6738 + Durvalumab | NSCLC | II | NCT03334617 | ||
| AZD6738 + Durvalumab | Advanced NSCLC with PD-1 ICI Resistance | II | NCT03833440 | ||
| AZD6738 + Durvalumab | Refractory Biliary Tract Cancer | II | NCT04298008 | ||
| AZD6738 + Olaparib | Relapsed SCLC | II | NCT03428607 | ||
| AZD6738 + Durvalumab | Metastatic Solid Tumors (Gastric Adenocarcinoma and Melanoma) | II | NCT03780608 | ||
| AZD6738 + Durvalumab/ Olaparib | Advanced Biliary Tract Cancer | II | NCT04298021 | ||
| Berzosertib (VX-970, M6620) | M6620 + Gemcitabine/Cisplatin/Carboplatin/ Irinotecan/Etoposide | Advanced Solid Tumors | I | NCT0215779297, 98, 146 | |
| Berzosertib + Lurbinectedin | Small Cell Cancers and High-Grade Neuroendocrine Cancers | I/II | NCT04802174 | ||
| VX-970 (M6620) + Topotecan | Small Cell Cancers | I/II | NCT0248709599 | ||
| M6620 + Carboplatin + Paclitaxel | Advanced Stage Solid Tumors | I | NCT03309150 | ||
| VX-970 + Veliparib + Cisplatin | Refractory Solid Tumors | I | NCT02723864 | ||
| M6620 + Cisplatin + Capecitabine + RT | Esophageal Adenocarcinoma and Other Solid Cancers | I | NCT03641547 | ||
| M6620 + Gemcitabine | Platinum-Resistant Recurrent Ovarian or Primary Peritoneal Fallopian Tube Cancer | II | NCT02595892147 | ||
| Berzosertib + Topotecan | Relapsed SCLC and Extrapulmonary Small-Cell Cancer | II | NCT03896503 | ||
| Berzosertib + Irinotecan | Advanced Solid Tumors | I | NCT02595931 | ||
| Berzosertib + Topotecan | Relapsed Platinum-resistant SCLC | II | NCT04768296 | ||
| Berzosertib + RT | Breast Cancer | I | NCT04052555 | ||
| Berzosertib + Sacituzumab Govitecan | SCLC and HR-Deficient Cancers Resistant to PARP inhibitors | I/II | NCT04826341 | ||
| Berzosertib + Cisplatin + RT | Locally Advanced HNSCC | I | NCT02567422 | ||
| Berzosertib + Pembrolizumab + Gemcitabine + Carboplatin | Chemotherapy-Naive Advanced NSCLC of Squamous Cell Histology | I/II | NCT04216316 | ||
| Berzosertib + Gemcitabine + Cisplatin | Metastatic Urothelial Carcinoma | II | NCT02567409 | ||
| Berzosertib + RT | Brain Metastases from Lung Cancer | I | NCT02589522 | ||
| Berzosertib + Carboplatin | Metastatic CRPC | II | NCT03517969 | ||
| Berzosertib + Carboplatin + Gemcitabine | First or Second Recurrence Platinum-Sensitive Epithelial Ovarian, Peritoneal, and Fallopian Tube Cancer | I | NCT02627443 | ||
| M6620 + Avelumab | Advanced Solid Tumors | I/II | NCT04266912 | ||
| M6620 + Avelumab + Carboplatin | PARP inhibitor-resistant Recurrent Ovarian, Primary Peritoneal, or Fallopian Tube Cancer | I | NCT03704467 | ||
| Elimusertib (BAY1895344) | BAY1895344 + Pembrolizumab | Advanced Solid Tumors | I | NCT04095273 | |
| Elimusertib (BAY1895344) + Irinotecan/Topotecan | Advanced Solid Tumors | I | NCT04514497 | ||
| Elimusertib (BAY1895344) + Cisplatin ± Gemcitabine | Advanced Solid Tumors | I | NCT04491942 | ||
| Elimusertib (BAY1895344) + FOLFIRI (Irinotecan, Fluorouracil, and Leucovorin) | GI Malignancies with a Focus on Metastatic Colorectal and Gastric/Gastroesophageal Cancers | I | NCT04535401 | ||
| BAY1895344 + Gemcitabine | Advanced Pancreatic and Ovarian Cancer | I | NCT04616534 | ||
| BAY1895344 + Niraparib | Recurrent Advanced Solid Tumors and Ovarian Cancer | I | NCT04267939 | ||
| ART0380 | ART0380 + Gemcitabine | Advanced or Metastatic Solid Tumors | I/II | NCT04657068 | |
| M1774 | M1774 + Niraparib | Metastatic or Locally Advanced Unresectable Solid Tumors | I | NCT04170153 | |
| M4344 (VX-803) | M4344 + Niraparib | PARP-Resistant Recurrent Ovarian Cancer | I | NCT04149145 | |
| M4344 + Carboplatin | Advanced Solid Tumors | I | NCT02278250 | ||
| RP3500 | RP3500 + Talazoparib | Advanced Solid Tumors | I/II | NCT04497116 | |
| CHK1/2 | Prexasertib (LY2606368) | Prexasertib (LY2606368) + LY3300054 (an anti-PD-L1 antibody) | Advanced Solid Tumors | I | NCT03495323123 |
| Prexasertib + Olaparib | Advanced Solid Tumors | I | NCT03057145 | ||
| Prexasertib + Cyclophosphamide/Gemcitabine | Refractory or Recurrent Group 3/Group 4 or Sonic Hedgehog Medulloblastoma | I | NCT04023669 | ||
| Prexasertib + LY3023414 (an inhibitor of the class I PI3K isoforms, mTOR, and DNA-PK) | TNBC | II | NCT04032080 | ||
| Prexasertib + Irinotecan | Relapsed or Refractory Desmoplastic Small Round Cell Tumor and Rhabdomyosarcoma | I/II | NCT04095221 | ||
| Prexasertib + Ralimetinib | Advanced or Metastatic Cancers | I | NCT02860780 | ||
| Prexasertib + RT + Cisplatin/Cetuximab | Locally Advanced HNSCC | I | NCT02555644 | ||
| Prexasertib (LY2606368) + Cytarabine + Fludarabine | Relapsed or Refractory Acute Myelogenous Leukemia and High-Risk Myelodysplastic Syndrome | I | NCT02649764 | ||
| Prexasertib + Cisplatin/Cetuximab/Pemetrexed/5-Fu/LY3023414 | Advanced and Metastatic Tumors | I | NCT02124148 | ||
| CHK1 | SRA737 | SRA737 + Gemcitabine (+ Cisplatin) | Advanced Solid Tumors | I/II | NCT02797977 |
| SCH900776 (MK-8776) | SCH900776 + Cytarabine | Relapsed Acute Myeloid Leukemia | II | NCT01870596 | |
| MK-8776 + Gemcitabine | Advanced Solid Tumors or Lymphoma | I | NCT00779584 | ||
| LY2603618 | LY2603618 + Pemetrexed + Cisplatin | NSCLC | I/II | NCT01139775 | |
| LY2603618 + Gemcitabine | Solid Tumors | I | NCT01341457 | ||
| LY2603618 + Gemcitabine | Pancreatic Cancer | I/II | NCT00839332148 | ||
| LY2603618 + Pemetrexed | Advanced or Metastatic NSCLC | II | NCT00988858 | ||
| WEE1 | Adavosertib (AZD1775, MK1775) | AZD1775 + cytarabine | Advanced Acute Myeloid Leukemia and Myelodysplastic Syndrome | II | NCT02666950 |
| AZD1775 + Cisplatin + Docetaxel | HNSCC | I | NCT02508246 | ||
| Adavosertib (MK1775) + Nab-Paclitaxel + Gemcitabine | Metastatic Pancreatic Adenocarcinoma | I/II | NCT02194829 | ||
| AZD1775 + Cisplatin + RT | Head and Neck Cancer (Hypopharynx Squamous Cell Carcinoma, Larynx Cancer, Oral Cavity Squamous Cell Carcinoma) | I | NCT03028766149 | ||
| Adavosertib (MK1775) + Gemcitabine | Ovarian Cancer, Primary Peritoneal Cancer, and Fallopian Tube Cancer | II | NCT02101775129 | ||
| AZD1775 + Irinotecan | Metastatic Colorectal Cancer | I | NCT02906059 | ||
| MK1755 + Gemcitabine + RT | Adenocarcinoma of the Pancreas | I/II | NCT02037230150 | ||
| Adavosertib + RT | Esophageal and Gastroesophageal Junction Cancer | I | NCT04460937 | ||
| Adavosertib + Cisplatin + RT | Cervical, Upper Vaginal, and Uterine Cancers | I | NCT03345784 | ||
| AZD1755 + Cisplatin + RT | HNSCC | I | NCT02585973 | ||
| AZD1775 + Carboplatin ± Paclitaxel | Advanced Solid Tumors | I | NCT02341456151 | ||
| Adavosertib + Irinotecan | Relapsed or Refractory Solid Tumors | I/II | NCT02095132 | ||
| Adavosertib + Temozolomide ± RT | Glioblastoma | I | NCT01849146 | ||
| AZD1775 + Olaparib | Refractory Solid Tumors | I | NCT02511795 | ||
| AZD1775 + Carboplatin + Paclitaxel | Squamous Cell Lung Cancer | II | NCT02513563 | ||
| AZD1775 + Cisplatin | TNBC | II | NCT03012477 | ||
| MK1775 + Paclitaxel + Carboplatin | Platinum Sensitive p53 Mutant Ovarian Cancer | II | NCT01357161 | ||
| MK-1775 + Gemcitabine/Cisplatin/Carboplatin | Advanced Solid Tumors | I | NCT00648648152 | ||
| AZD1775 + MEDI4736 (Durvalumab) | Muscle Invasive Bladder Cancer | I | NCT02546661 | ||
| AZD1775 (Adavosertib) + MEDI4736 (Durvalumab) | Advanced Solid Tumours | I | NCT02617277 | ||
| AZD1775 + Carboplatin | Children, Adolescents and Young Adults with Refractory or Recurrent Malignancies | I/II | NCT02813135 | ||
| Adavosertib + Gemcitabine/Paclitaxel/Carboplatin/Pegylated Liposomal Doxorubicin | Platinum-Resistant Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | II | NCT02272790 | ||
| Adavosertib + Carboplatin | Advanced Malignant Solid Neoplasm | II | NCT01827384 | ||
| Adavosertib + Olaparib | Advanced Malignant Solid Neoplasm | I | NCT04197713 | ||
| Adavosertib + Olaparib | Ovarian Cancer | II | NCT03579316 | ||
| Adavosertib + RT | Newly Diagnosed Diffuse Intrinsic Pontine Gliomas in Children | I | NCT01922076 | ||
| ATR or WEE1 | Ceralasertib (AZD6738)/Adavosertib (AZD1775) | Ceralasertib/Adavosertib + Olaparib | Metastatic TNBC | II | NCT03330847 |
| Olaparib (AZD2281) + AZD1775/AZD6738 | Advanced Solid Tumors | II | NCT02576444 | ||
| AZD1775 + Carboplatin; AZD6738 + Olaparib | Platinum Refractory Extensive-Stage SCLC | II | NCT02937818 |
- CRPC, castration-resistant prostate cancer; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small cell lung cancer; RT, radiotherapy or radiation therapy; SCLC, small-cell lung cancer; TNBC, triple-negative breast cancer.
6.2 Checkpoint kinase 1/2: Function and inhibition
CHK1 is a serine/threonine protein kinase and the major downstream effector of ATR. CHK1 is recruited to RPA-covered ssDNA by phosphorylated claspin, which enables ATR to phosphorylate CHK1 directly at serines Ser317 and Ser345.105 CHK1 is then autophosphorylated and activated. The activated CHK1 goes on to phosphorylate downstream targets CDC25A, CDC25C, and WEE1, inactivating the CDC25 phosphatase proteins and activating WEE1.83 Phosphorylated CDC25A is subsequently degraded by proteosomes, which leads to a decrease in CDK2 activity in S-phase; while phosphorylation of CDC25B and CDC25C promotes their degradation and the inhibition of CDK1/cyclin B kinases, causing G2/M cell cycle arrest and allowing more time to repair the damaged DNA before entering mitosis.106 Most cancer cells have lost G1 checkpoint function, making them extremely reliant upon S and G2 checkpoints.
CHK2, in contrast, is a serine/threonine protein kinase and the downstream effector of ATM.105 In addition to direct activation, ATM can, through the activation of CHK2, indirectly activate p53, followed by activation of p21.107 ATR/CHK1 and ATM/CHK2 pathways have cross-talk interactions. For example, they both activate p53, inactivate CDC25A, and regulate HR factors. Targeting CHK2 alone in cancer treatment, however, has not been thoroughly studied. Early in 2009, Jobson et al.108 reported a CHK2 inhibitor PV1019 that inhibits autophosphorylation and shows synergistic antiproliferative activity with topotecan, camptothecin, and radiation in human cancer cell lines. Anderson et al.109 also reported a potent and selective CHK2 inhibitor CCT241533 that potentiates the cytotoxicity of two different PARP inhibitors. A phase I study (ClinicalTrials.gov Identifier: NCT04678102) using PHI-101, an inhibitor of CHK2 in patients with platinum-resistant or refractory ovarian cancer is ongoing. More studies are needed to elucidate its potential role in cancer treatment.
AZD7622 is an ATP-competitive and selective CHK1/CHK2 inhibitor, prohibiting the S and G2 checkpoints and, thus, hindering DNA repair. In preclinical trials, AZD7622 has been shown to enhance the activity of DNA-damaging agents, as the activities of gemcitabine and topotecan are enhanced in combination with AZD7622 in p53-mutant SW620 colon cancer cells and p53-mutant MDA-MB-231 breast carcinoma cells in vitro, respectively,110 and AZD 7762 sensitizes MiaPaCa-2 human pancreatic ductal adenocarcinoma cells to radiation with the abrogation of the G2 checkpoint and inhibition of HR,111 likely through inhibition of RAD51. More preclinical studies have shown the efficacy of the combination of AZD7762 and gemcitabine in urothelial carcinoma112 and aggressive KRAS-driven LKB1-deficient lung adenocarcinoma.113 Another oral CHK1 inhibitor, SRA737, when combined with low dose gemcitabine, enhances the effect of anti-PD-L1 in a small cell lung cancer model by significantly increasing antitumorigenic CD8+ cytotoxic T-cell, dendritic cell, and M1 macrophage populations through activation of STING and interferon signaling and induction of expression of PD-L1 and inflammatory chemokines.114 Moreover, in an unbiased large-scale siRNA screen, SRA737 is found to be synthetically lethal in combination with subunits of the B-family of DNA polymerases, POLA1, POLE, and POLE2 in human non-small cell lung cancer and colorectal cancer cell lines.115
Prexasertib (LY2606368) is a potent inhibitor of CHK1 and to a lesser extent, CHK2. Upon inhibition of CHK1 by LY2606368, the downstream CDC25A and CDK2 activity is inhibited, which subsequently increases the replication forks and adds to the genomic instability of the cells.116 A preclinical study has shown that prexasertib reverses restored HR and replication fork stability, and exhibits monotherapy and olaparib-combined synergistic activity in PARP inhibitor-resistant high-grade serous ovarian carcinoma (HGSOC), both in patient-derived xenografts and cell line models.117 In another preclinical study, pharmacological inhibition of CHK1 by prexasertib downregulates BRCA1 and RAD51 levels, and prexasertib allows olaparib-treated triple-negative breast cancer to enter mitosis without repairing damage at either cell-cycle checkpoint, resulting in mitotic catastrophe and cell death.118 Other preclinical studies have demonstrated that prexasertib enhances cytotoxicity of cisplatin and radiation in primary patient-derived osteosarcoma119 and head and neck squamous cell carcinoma.120 Phase I (ClinicalTrials.gov Identifier: NCT01115790, squamous cell carcinoma)121 and II (ClinicalTrials.gov Identifier: NCT02203513, HGSOC)122 studies have demonstrated the safety profile and single-agent activity in patients with different types of cancer. The latter study indicated that neutropenia was observed in 79% of the treated patients and was the most frequent toxicity. In an open-label phase I study (ClinicalTrials.gov Identifier: NCT03495323), prexasertib in combination with anti-PD-L1 antibody LY3300054 was tolerable and demonstrated preliminary activity in CCNE1-amplified HGSOC, with evidence showing activation of cytotoxic T-cells in patient blood samples.123 Among the 17 enrolled patients, three patients with CCNE1-amplified HGSOC achieved PR, and one such patient remained SD. Ongoing clinical studies of combination therapies based on prexasertib and other CHK1 inhibitors are listed in Table 3.
6.3 WEE1: Function and inhibition
WEE1 is a tyrosine kinase involved in cell-cycle regulation in the G2-M cell-cycle checkpoint. In higher eukaryotic cells, the cell cycle is precisely regulated by different cyclins and CDKs.124 WEE1 is activated by the ATR-CHK1 signaling pathway, after which it phosphorylates CDK1 and inhibits its activity.125 As is described in the ATR/CHK1 pathway above, CDK1/cyclin B activity is required at G2-M transition, and hence, loss of CDK1 activity leads to cell-cycle arrest at G2-M transition before entry into mitosis. WEE1 is highly expressed in a variety of cancer cells, indicating their heavy reliance on the G2-M checkpoint and potential to be targeted by WEE1 inhibitors.126
Adavosertib (MK1775 or AZD1775) is a highly selective ATP-competitive inhibitor of WEE1. The first report of AZD1755 single-agent activity was a phase I study in patients with refractory solid tumors (ClinicalTrials.gov Identifier: NCT01748825), which for the first time demonstrated its MTD, pharmacokinetics, biochemical results, as well as observed results of common toxicities of myelosuppression and diarrhea, and dose-limiting toxicities of supraventricular tachyarrhythmia and myelosuppression.127 A phase II study (ClinicalTrials.gov Identifier: NCT01164995) provides proof that AZD1775 enhances carboplatin efficacy in TP53-mutated tumors, and demonstrates that of the 21 evaluable patients after treatment, eight patients (38%) showed a PR as the best response, and one patient (5%) had a complete response, resulting in an overall response rate of 43% (95% CI: 22 to 66%) with manageable toxicity, mainly including fatigue, nausea, and vomiting, thrombocytopenia, and diarrhea.128 Recently, Lheureux et al.129 have discovered in a phase II trial (ClinicalTrials.gov Identifier: NCT02101775) that adavosertib administered in combination with gemcitabine significantly extends progression-free survival and overall survival in women patients with platinum-resistant or platinum-refractory advanced HGSOC. The median progression-free survival was 4.6 months (95% CI: 3.6 to 6.4) in the combination group versus 3.0 months (1.8 to 3.8) in the placebo plus gemcitabine group, with the hazard ratio at 0.55 (0.35 to 0.90, log-rank p = 0.015). And the median overall survival at data cutoff was 11.4 months (8.2 to 16.5) versus 7.2 months (5.2 to 13.2) with the hazard ratio at 0.56 (0.35 to 0.91, log-rank p = 0.017). The most frequent grade 3 or worse adverse events in the combination group were neutropenia (62 versus 30% in the control group) and thrombocytopenia (31 versus 6%). The positive result makes adavosertib another promising DDR inhibitor for clinical use and warrants application of the combined therapeutic approach in other tumor types with high replication stress. More clinical trials of adavosertib-based combination therapies are ongoing and listed in Table 3.
7 POTENTIALLY TARGETABLE DDR PATHWAYS
Apart from the DDR pathways elaborated above, more DDR pathways participate in DNA repair and have the potential to be targeted in cancer treatment. The pathways below are undruggable or not optimized in their use clinically, and thus, will be introduced briefly.
DNA MMR is a conserved pathway that repairs DNA by removing mismatched nucleotides or insertion or deletion loops with the help of MMR proteins. In eukaryotic cells, the MMR mechanism starts with mismatch recognition, followed by protein recruitment, excision, and gap filling. Loss of the MMR function may lead to genomic instability and a predisposition to cancer.130 So far, no clinical drug targeting the MMR mechanism has been developed, probably due to its undruggability.131
NER is a versatile mechanism that primarily removes helix-distorting DNA lesions, such as bulky base adducts and ultraviolet photo-products,6 and is composed of DNA lesion recognition, dual incisions with the removal of a short oligonucleotide, and gap filling using the undamaged complementary strand as the template.132 Similar to BER, NER cuts one strand of the DNA molecule and connects the broken ends according to the undamaged strand, which contributes to the resistance of some DNA-damaging agents such as cisplatin.133 van Midwoud and Sturla134 demonstrated that inhibiting the NER pathway can reduce the use of anticancer drug acylfulvene required to kill HT29 colon cancer cells by twofold. A recent study showed that NER-defective cells hyperactive cell-cycle checkpoint, MK2 signaling, particularly in response to platinum-based chemotherapy, and that combined depletion of MK2 and XPA (a critical common scaffold required for the NER pathway) revealed dramatic synergistic killing of p53-defective KP7B lung adenocarcinoma cells in response to cisplatin treatment, both in culture and in mouse models,135 indicating the huge potential of NER inhibition in cancer treatment.
TLS is the direct replication of DNA damage, an important component of DNA damage tolerance to resolve replication stress. TLS involves specialized DNA polymerases to promote the direct bypass of DNA lesions that block replication fork progression such as DNA adducts and unusual template structures.136 Although TLS polymerase gene deficiency may be linked to cancer, downregulation of TLS sensitizes cancer cells to chemotherapeutics, notably the platinum-based DNA cross-linking agents.137 JH-RE-06 is a small-molecule inhibitor of TLS by preventing recruitment of mutagenic polymerase ζ that has been found to enhance cisplatin-induced toxicity in both cultured human and mouse cell lines and suppress tumor growth in xenograft human melanomas in mice when coadministered with cisplatin.138 More studies are needed to explore the underlying mechanisms and feasibility of TLS inhibition in cancer treatment.
FA is an autosomal and less commonly, X-linked recessive genetic disorder caused by biallelic mutations in FA genes. The FA pathway is a biochemical network that participates in DNA repair, replication, and other cellular processes and is activated by interstrand crosslinks during the S phase, which is subsequently repaired by this pathway.139 Alterations of the FA genes contribute to cancer development, while targeting the FA pathway may be a prospective method for cancer treatment in terms of eliminating drug resistance and achieving SL with other genes.140 The FA pathway protects cells from R-loop accumulation and genomic instability and regulates transcription of some genes, which contribute to cancer development. Upregulation of FA gene expression is pervasive in different cancers and has been found to be associated with chemotherapy resistance, thus, the potential to be targeted in combination therapies to achieve SL.
8 LIMITATIONS AND FUTURE PERSPECTIVES
The agents that inhibit the DDR pathways can demonstrate synergistic effects with traditional antineoplastic drugs, and also adverse effects that may restrict their use. In normal cells, DNA molecules undergo physiological lesions and need the repair pathways to maintain their normal state. Blocking of the DDR pathways may impede the repair procedure and damage or even kill these cells, presenting as TEAEs and dose-limiting toxicities in clinical use. From the results of finished clinical trials, most of the DDR-inhibiting agents are well tolerated in early phase trials, but a variety of adverse events still exist and call for attention. Most of the clinical trials have demonstrated that neutropenia is one of the most common adverse drug effects of DDR inhibitors, indicating bone marrow suppression that accord with chemotherapy and may cause dose-limiting toxicity in combination therapies. Moreover, as DDR pathways protect cells from DNA-damaging agents and potential carcinogenic mutations, blocking these pathways may magnify genomic instability and increase the risk of another cancer. More studies are needed to elucidate the toxicities and their mechanisms of different DDR inhibitors on normal tissues. Future DDR inhibitor development should focus not only on better therapeutic effects but also on milder adverse events especially when combined with other antitumor treatments.
DNA damage-inducing therapies, such as chemotherapy, can induce cellular senescence, a cellular response that maintains tissue homeostasis and prevents tumorigenesis.141 Cellular senescence is closely related to DDR pathways, which help initiate DNA repair and activate cell-cycle checkpoints to maintain a permanent cell-cycle arrest. Senescence in tumor cells may enhance sensitivity to DDR inhibitors, while senescence in normal cells leads to heavier reliance on DDR pathways to maintain homeostasis. Moreover, cellular senescence promotes local and systemic inflammatory responses which can cause or exacerbate the side-effects of chemotherapy.142 Older patients may have accumulated more senescent cells as well as DNA damage burden, which preferably activates DDR pathways. To date, no clear evidence has demonstrated the correlation between responses to DDR inhibitors and patients’ age on the basis of DNA damage-inducing therapies. Therefore, further studies are warranted to find out their correlation.
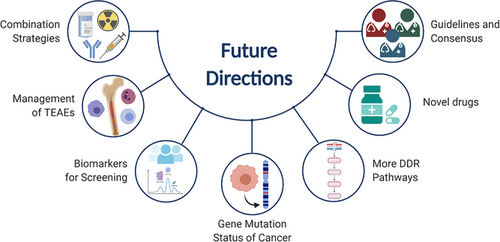
Although several common DDR inhibition-based combination strategies are undergoing clinical trials, no normative strategies have been confirmed. To fully exploit DDR inhibitors and promote the combination therapies, future studies should be oriented toward some key directions, as illustrated in Figure 4. More preclinical and clinical studies are needed to find out appropriate combination strategies of DDR inhibitors, dosages in the combination, chronological order of treatment administration, and the management of TEAEs in combination. Since drug resistance is common in cancer treatment, new combination strategies can provide novel methods to overcome drug resistance and increase overall survival. As PARP inhibitors are specifically effective in patients with BRCA mutations, other DDR inhibitors should have superiority in particular patients. Therefore, it is necessary to find out useful biomarkers and gene mutation status to screen the potential patients who may or may not benefit from a specific combination strategy. In addition, some of the DDR pathways have not been exploited in cancer treatment. Future studies can dig deeper into these pathways and develop novel agents to target them. We believe that DDR inhibitors will be more widely used in cancer treatment and more combination strategies will be developed.
9 CONCLUSIONS
Cancer treatment is a complex and perplexing issue. Each new therapy was subjected to extensive testing prior to being allowed into clinical practice. With a growing understanding of DDR's role in cancer, its potential value in cancer treatment became apparent. PARP inhibitors paved the way for the clinical use of DDR inhibitors, which was quickly followed by the discovery of other DDR inhibitors, with multiple clinical trials currently ongoing. Using PARP inhibitors as an example, innovative DDR inhibitors may employ synthetic lethal techniques to increase their antitumor properties. Apart from the continuously improving chemotherapy and radiotherapy regimens, immunotherapeutic drugs are emerging as a promising partner for combination therapies. Along with PARP inhibitors, agents targeting DNA-PK, ATR, CHK1, and WEE1 are being evaluated, with promising results in combination therapies. Additional studies are required to identify biomarkers that can be used to guide the screening of patients and predict the response and resistance. Although more work needs to be done, we may anticipate that DDR inhibitors and combination strategies will play a greater role in cancer treatment in the future.
ACKNOWLEDGMENTS
We would like to thank the authors of the primary studies. The figures in this article were created using BioRender. This work was supported by Zhejiang Provincial Natural Science Foundation of China under Grant No. LQ22H160003, National Natural Science Foundation of China (81827804), Zhejiang Clinical Research Center of Minimally Invasive Diagnosis and Treatment of Abdominal Diseases (2018E50003), and Key Research and Development Project of Zhejiang Province (2018C03083).
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
Tianen Chen: Conceptualization; Data curation; Writing–original draft. Suparat Tongpeng: Writing–original draft. Ziyi Lu: Writing–original draft. Win Topatana: Writing–original draft. Sarun Juengpanich: Writing–original draft. Shijie Li: Writing–original draft. Jiahao Hu: Writing–original draft. Jiasheng Cao: Writing–original draft. Cheeshin Lee: Writing–original draft. Yitong Tian: Writing–original draft. Mingyu Chen: Project administration; Writing–review & editing. Xiujun Cai: Project administration; Writing–review & editing
Open Research
DATA AVAILABILITY STATEMENT
The data of clinical trials with a ClinicalTrials.gov Identifier were obtained from https://clinicaltrials.gov/.




