Journal list menu
Export Citations
Download PDFs
Issue Information
Editor's Choice
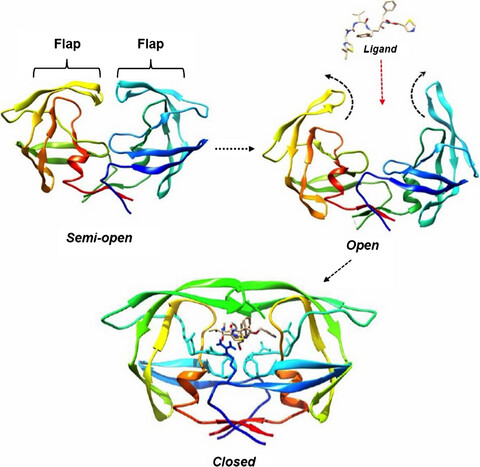
Flap Dynamics in Aspartic Proteases: A Computational Perspective
- Pages: 159-177
- First Published: 13 February 2016
Research Articles
Discovery of Novel Allosteric Eg5 Inhibitors Through Structure-Based Virtual Screening
- Pages: 178-187
- First Published: 10 February 2016

We explored a novel allosteric site of kinesin Eg5 and identified new Eg5 inhibitors using structure-based virtual screening. The identified compounds significantly induced monopolar spindle formation in colorectal cancer HCT116 cells and suppressed cancer cell viability and colony formation. Our findings reveal a new allosteric regulation mechanism of Eg5 and a novel drug targeting site for cancer therapy.
Some Anticancer Agents Act on Human Serum Paraoxonase-1 to Reduce Its Activity
- Pages: 188-196
- First Published: 13 February 2016
Human serum paraoxonase (hPON1) protects low-density lipoproteins against oxidative stress and prevent atherosclerosis development. Anticancer agents have cardiotoxic effects, and this situation can lead to significant complications. The aim was to evaluate the in vitro effects of some of the anticancer agents such as cetuximab, paclitaxel, etoposide, docetaxel, and ifosfamide on the activity of hPON1 in this study. These drugs reduced the activity of hPON1. Consequently, inhibition of hPON1 by these anticancer agents may explain some of the cardiotoxic actions of these drugs.
Discovery of New Substrates for LuxAB Bacterial Bioluminescence
- Pages: 197-208
- First Published: 19 February 2016

In this article, four novel substrates with long halftime have been designed and synthesized successfully for luxAB bacterial bioluminescence. After in vitro and in vivo biological evaluation, these molecules can emit obvious bioluminescence emission with known bacterial luciferase, thus indicating a new promising approach to developing the bacterial bioluminescent system.
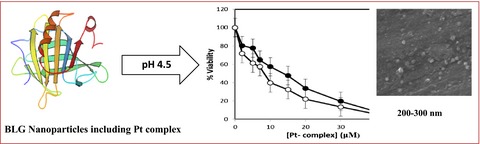
β-lactoglobulin–pectin Nanoparticle-based Oral Drug Delivery System for Potential Treatment of Colon Cancer
- Pages: 209-216
- First Published: 19 February 2016
Glycolipid-based TLR4 Modulators and Fluorescent Probes: Rational Design, Synthesis, and Biological Properties
- Pages: 217-229
- First Published: 19 February 2016
We present here the computer-aided rational design and the synthesis of new small molecule TLR4 modulators: fluorescent compounds 1 and 2 and homodimers 3 and 4. These non-toxic molecules were active in inhibiting LPS-triggered TLR4 stimulus in HEK-Blue cells and molecule 1 was used as fluorescent label for LPS-binding proteins in confocal microscopy experiments on cells.
Enhanced Cytotoxicity to Cancer Cells by Codelivery and Controlled Release of Paclitaxel-loaded Sirolimus-conjugated Albumin Nanoparticles
- Pages: 230-240
- First Published: 23 February 2016
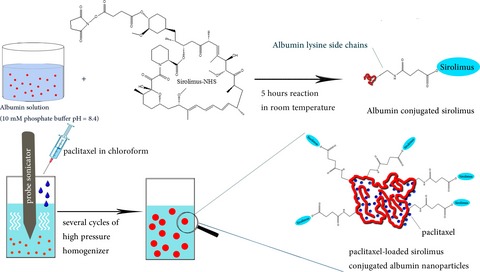
The paclitaxel-loaded sirolimus-conjugated albumin nanoparticles were prepared to enhance cytotoxicity of paclitaxel to cancer cells. The cellular studies showed that paclitaxel concentration ratio to sirolimus and time of sirolimus release after paclitaxel were two important factors in cytotoxic effects. We guess that when cancer cell lines arrest in G2-M by anticancer drugs like paclitaxel, Akt activates mTOR to make cells continue living, then inhibiting mTOR can enhance anticancer effects.
Arylazolyl(azinyl)thioacetanilides: Part 19: Discovery of Novel Substituted Imidazo[4,5-b]pyridin-2-ylthioacetanilides as Potent HIV NNRTIs Via a Structure-based Bioisosterism Approach
- Pages: 241-253
- First Published: 23 February 2016
![Arylazolyl(azinyl)thioacetanilides: Part 19: Discovery of Novel Substituted Imidazo[4,5-b]pyridin-2-ylthioacetanilides as Potent HIV NNRTIs Via a Structure-based Bioisosterism Approach](/cms/asset/685ca52b-736b-4d35-b7de-d504dd42df32/cbdd12751-toc-0001-m.jpg)
A series of novel imidazo[4,5-b]pyridin-2-ylthioacetanilides were designed, synthesized, and evaluated for their antiviral activities through combining bioisosteric replacement and structure-based drug design. Almost all of the title compounds displayed a moderate to excellent activities against wild-type (wt) HIV-1 strain with EC50 values ranging from 0.059 to 1.41 μm in a cell-based antiviral assay. Preliminary structure–activity relationships (SARs) and molecular modeling studies were detailedly discussed, which may provide valuable insights for further optimization.
The Synthesis of 1,3,5-triazine Derivatives and JNJ7777120 Analogues with Histamine H4 Receptor Affinity and Their Interaction with PTEN Promoter
- Pages: 254-263
- First Published: 01 March 2016
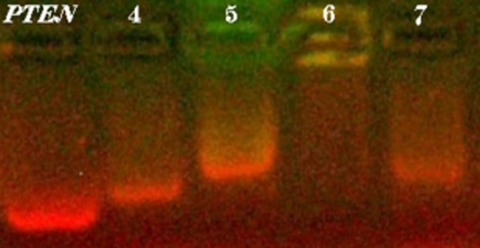
PTEN has been described as the most frequently mutated tumor suppressor gene in human cancer. We described here the synthesis of new H4 receptor ligands and their interaction with the promoter of PTEN gene demonstrated by using electrophoretic mobility shift assay. Several compounds based on the well-known H4R antagonist JNJ7777120 structure showed high effect on PTEN promoter mobility. However, no correlation between PTEN interaction and anti-neuroblastoma activity was found. Moreover, one triazine derivative with the highest H4R affinity was shown as a promising and non-cytotoxic lead structure.
Synthesis and Biological Evaluation of 3, 8-dimethyl-5-isopropylazulene Derivatives as Anti-gastric Ulcer Agent
- Pages: 264-271
- First Published: 03 March 2016
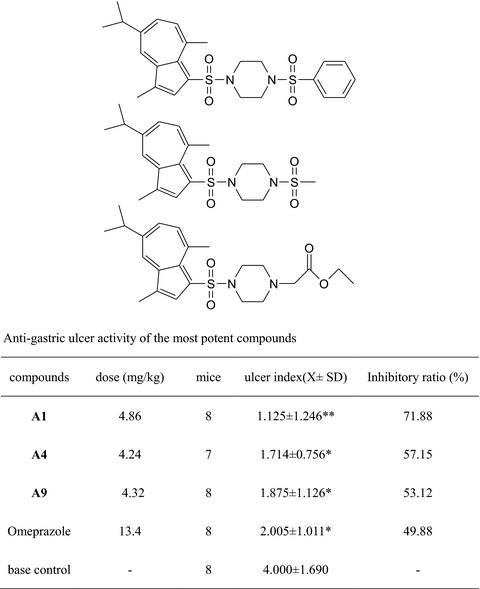
Two series of GA derivatives were synthesized and evaluated for their anti-gastric ulcer activity. The data obtained from in vivo testing of these compounds in a rodent ethanol-induced stomach injury model showed that A1, A4, and A9 (ulcer index: 1.125 ± 1.246**, 1.714 ± 0.756*, 1.875 ± 1.126*) exhibited better anti-gastric ulcer activity than the positive control Omeprazole (2.005 ± 1.011*).
The Binding Mode Prediction and Similar Ligand Potency in the Active Site of Vitamin D Receptor with QM/MM Interaction, MESP, and MD Simulation
- Pages: 272-280
- First Published: 06 March 2016
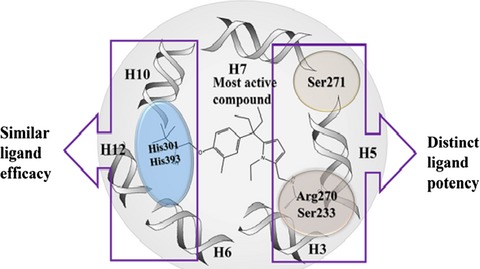
Different in silico approaches were applied to study the specific interactions of non-secosteroidal ligands in VDR active site. The article summarizes the importance of similar ligand efficacy and the distinct ligand potency in the VDR active site. The results could be useful for the designing of potent agonists in the lead optimization phase of drug discovery against VDR.
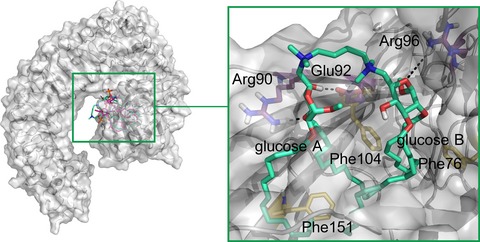
Insights into Medium-chain Acyl-CoA Dehydrogenase Structure by Molecular Dynamics Simulations
- Pages: 281-292
- First Published: 15 March 2016
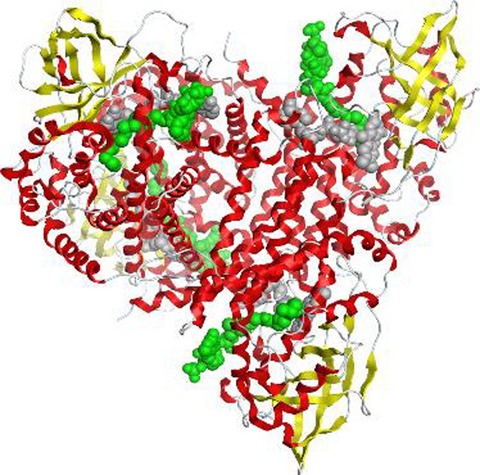
The medium-chain acyl-CoA dehydrogenase (MCAD) is an enzyme that catalyzes the first step of mitochondrial fatty acid β-oxidation pathway. Herein, we analyzed the structural stability and dynamic behavior of MCAD wild-type by means of molecular dynamics studies. Our results revealed that the FAD cofactor has an important structural role on the tetramer stability and also in maintaining the volume of the catalytic pockets. We confirmed that the presence of fatty-acyl substrate changes the dynamics of the catalytic pockets favoring the reaction mechanism.
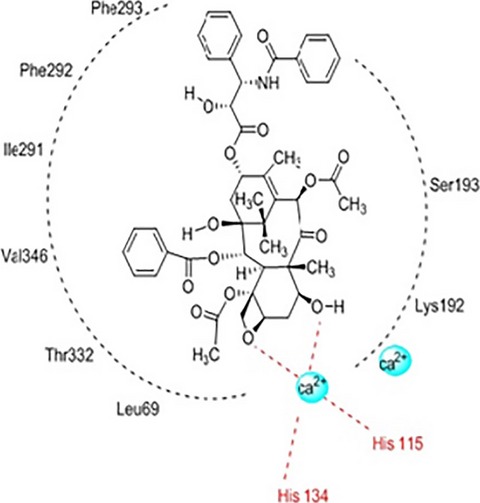
Bioassay-Guided Isolation and Structural Modification of the Anti-TB Resorcinols from Ardisia gigantifolia
- Pages: 293-301
- First Published: 15 March 2016
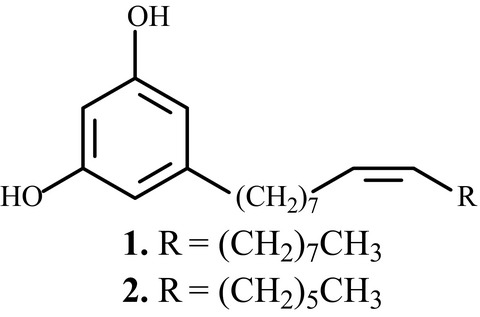
We have identified two antitubercular (anti-TB) 5-alkylresorcinols, 5-(8Z-heptadecenyl) resorcinol (1) and 5-(8Z-pentadecenyl) resorcinol (2) through bioassay-guided separation from the leaves and stems of the medicinal plant Ardisia gigantifolia, and further synthesized 15 derivatives (3-17) based on these two natural products. These compounds were evaluated for their anti-TB activity against Mycobacterium tuberculosis H37RV.
Selective Inhibition of PTP1B by Vitalboside A from Syzygium cumini Enhances Insulin Sensitivity and Attenuates Lipid Accumulation Via Partial Agonism to PPARγ: In Vitro and In Silico Investigation
- Pages: 302-312
- First Published: 15 March 2016
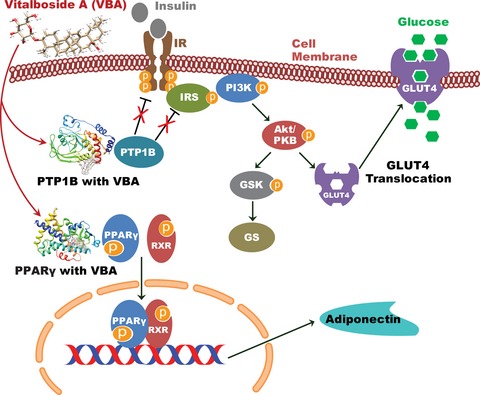
Bifunctional compound was purified and identified as Vitalboside A (VBA), exhibiting antidiabetic and anti-adipogenic activity. Biochemical and docking analysis confirms VBA as an allosteric non-competitive inhibitor of PTP1B. Inhibitor studies and Western blot analysis revealed that VBA is a potential insulin-sensitizing candidate which increases glucose transport (GLUT 4) in a PI3K/AKT-dependent manner. In silico and in vitro studies showed partial transactivation of peroxisome proliferator-activated receptor γ, with an increase in adiponectin secretion, confirming the anti-adipogenic potential of VBA.