Insights into Medium-chain Acyl-CoA Dehydrogenase Structure by Molecular Dynamics Simulations
Abstract
The medium-chain acyl-CoA dehydrogenase (MCAD) is a mitochondrial enzyme that catalyzes the first step of mitochondrial fatty acid β-oxidation (mFAO) pathway. Its deficiency is the most common genetic disorder of mFAO. Many of the MCAD disease-causing variants, including the most common p.K304E variant, show loss of function due to protein misfolding. Herein, we used molecular dynamics simulations to provide insights into the structural stability and dynamic behavior of MCAD wild-type (MCADwt) and validate a structure that would allow reliable new studies on its variants. Our results revealed that in both proteins the flavin adenine dinucleotide (FAD) has an important structural role on the tetramer stability and also in maintaining the volume of the enzyme catalytic pockets. We confirmed that the presence of substrate changes the dynamics of the catalytic pockets and increases FAD affinity. A comparison between the porcine MCADwt (pMCADwt) and human MCADwt (hMCADwt) structures revealed that both proteins are essentially similar and that the reversion of the double mutant E376G/T255E of hMCAD enzyme does not affect the structure of the protein neither its behavior in simulation. Our validated hMCADwt structure is crucial for complementing and accelerating the experimental studies aiming for the discovery and development of potential stabilizers of MCAD variants as candidates for the treatment of MCAD deficiency (MCADD).
The medium-chain acyl-CoA dehydrogenase (MCAD; EC 1.3.8.7) is a mitochondrial enzyme that belongs to the acyl-CoA dehydrogenases family of flavoproteins and catalyzes the first step of the mitochondrial fatty acid β-oxidation (mFAO) pathway, a dehydrogenation reaction in the Cα-Cβ carbon atoms of medium-chain fatty-acyl substrates 1. The mature MCAD is a homotetramer having each monomer (Mw ≈ 44 kDa) three structural domains: the N-terminal α-domain (from N-terminus to L129) and the C-terminal α-domain (from E240 to C-terminus) flanking an intermediate β-sheet domain (from M130 to G239) (Figure 1A). The N- and C-terminal α-domains consist of six α-helices (A-F and G-L, respectively) that together seem to form a single α-domain. The β-domain is located at the protein surface and is composed by two orthogonal β-sheets 2-4. The Flavin adenine dinucleotide (FAD) is the natural cofactor of MCAD which binds non-covalently to the active site, being critical for enzyme's function. It also seems to have a structural role as it has been described that upon incorporation of FAD in each monomer, dimerization is promoted followed by the assembly of two homodimers into the final tetrameric form 1, 4. The tetramer may be considered a dimer of dimers and the interface between them involves groups of four α-helices from the C-terminal α-domain of each monomer (protein core) (Figure 1B).
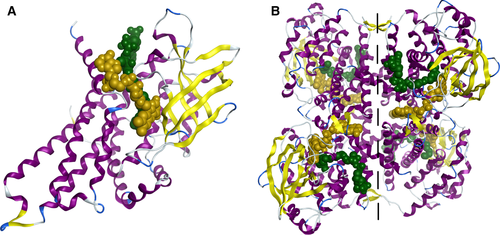
The MCAD protein has four catalytic pockets being each pocket formed by the two monomers of the same dimer 3, 4. The riboflavin moiety of FAD is located between the β-domain and the C-terminal α-domain of one monomer with the sinister (si)-face of flavin ring directed to the β-domain. The pyrophosphate portion of FAD lies between the β-domain of one monomer and the C-terminal α-domain of the other, while the adenosine group is located at the interface between the two monomers of the dimer.
The 3′-phosphoadenosine of the CoA moiety is located at the interface between the two neighboring monomers. The Cα-Cβ bond of the fatty-acyl substrate is inserted between the carboxylate group (side chain) of the glutamate residue 376 (E376; catalytic residue) and the flavin ring of FAD, which favors the α, β-dehydrogenation reaction 3, 4.
Mechanistically, the catalytic residue E376 removes the α-hydrogen of the acyl moiety of the substrate as a proton and the β-hydrogen is transferred as a hydride onto the N5 of the flavin ring of FAD, yielding a trans-Δ2-enoyl-CoA derivative and FADH2, respectively (Figure S1) 5.2
The MCAD deficiency is the most common genetic disorder of mFAO 1. It is characterized by recurrent metabolic crisis that may lead to coma and unexpected death triggered by situations of catabolic stress as prolonged fasting or fever episodes 1, 6. Most of the MCAD disease-causing mutations result from single amino acid changes (missense mutations) in the human MCAD (hMCAD) sequence and lead to variant proteins, including the most common p.K304E variant, which have been related with protein misfolding and/or misassembling with enzyme's loss of function (conformational disorder) 7-10. Currently, there is no approved pharmacological treatment for MCAD deficiency (MCADD). The long-term treatment of MCADD patients consists mainly in the avoidance of fasting through frequent feeding 7, 11.
Presently, all major work developed on MCAD enzyme is experimental, mainly focused on its catalytic mechanism and on the structural/functional characterization of hMCAD wild-type (wt) and its variant proteins 5, 7, 8, 12-25. The MCAD redox mechanism is reported in many in vitro 5, 25-28 and in silico 29-31 studies. Nevertheless, the folding processes of MCAD variants causing MCADD are not fully understood.
Molecular dynamics (MD) simulations have been shown to be a suitable computational approach to study the protein folding/unfolding processes at atomic level complementing the experimental studies 32, 33. In the present work, MCAD was studied using MD simulations, to provide insights into the structural stability and dynamic behavior of the wild-type form of MCAD (MCADwt) and validate a structure that would assist further studies on MCAD variants.
Until the moment no crystallographic structure of the hMCADwt in the presence of the FAD cofactor and the fatty-acyl substrate is available. The human MCAD structures accessible in the Protein Data Bank (PDB) were obtained either with the protein modified with chimeric point mutations, namely in the catalytic residue E376 [PDB ID:1EGC 34], in complex with the ETF flavoprotein [PDB ID: 1T9G 26], or more recently, but also without the fatty-acyl substrate, the p.K304E variant (PDB ID: 4P13). In fact, due to the lack of reliable structural data on hMCADwt, the crystallographic structure of porcine MCADwt (pMCADwt) is commonly used for predictions on the potential impact of mutations found in MCAD deficient patients 13-15. Herein, the crystallographic structure of the pMCAD enzyme [PDB ID: 1UDY 4], in complex with the FAD cofactor and the 3-thiaoctanoyl-CoA (SC8-COA) substrate, sharing more than 90% of residues identity with the hMCAD, was primarily used to model MCAD enzyme. However, to compare and validate the results obtained from these simulations, the same approach was applied to characterize the hMCADwt protein in the presence of the FAD and the fatty-acyl substrate.
Thus, the E376G/T255E mutations within the crystallographic structure of hMCAD protein [PDB ID: 1EGC 34], in complex with FAD and the octanoyl-CoA (C8-COA) substrate were reversed and the resulting hMCADwt structure also thoroughly studied by MD simulations.
All simulations were performed during 100 ns, and for each wt protein, three systems were built: APO (no substrate, no FAD), FAD (no substrate, with FAD), and LIPID (with substrate and FAD).
Methods
Systems’ preparation
The starting coordinates of pMCADwt were obtained from the crystallographic structure of the Sus scrofa (pig) MCAD protein [PDB ID: 1UDY 4], in complex with FAD and SC8-COA substrate. The hMCADwt protein was obtained by reversing the E376G/T255E double mutation in the crystallographic structure with PDB ID as 1EGC, where the protein is in complex with FAD and C8-COA substrate. For each wt protein, three different systems were built: APO (no substrate, no FAD), FAD (no substrate, with FAD) and LIPID (with substrate and FAD). The MCAD structures were prepared for the MD simulations by firstly removing all the water molecules within the crystallographic structures, followed by their protonation at T = 310 K and pH = 7.4 using the Protonate 3D module available in the moe 2013.081 software. To use these structures with the GROMOS96 35, 36 53a6 37 force field, all the non-polar hydrogens were removed using the moe 2013.08 software. The protein topologies were generated according with the GROMOS96 53a6 force field by the pdb2gmx module available in the gromacs 4.6.3 38-41 package. The topologies of the FAD, SC8-COA, and C8-COA were obtained using the Automated Topology Builder and Repository (ATB) 42, 43 web server according to the selected force field. Each protein was kept in a cubic simulation box with dimensions xyz of 13.1 × 13.1 × 13.1 nm (pMCAD) and 12.9 × 12.9 × 12.9 nm (hMCAD). To avoid interactions with the respective periodic images and to allow periodic boundary conditions (PBC), each protein was centered at a distance of 1.0 nm from the box edge.
The systems constructed for pMCADwt protein were solvated with 66462 (APO), 66354 (FAD) and 66208 (LIPID) water molecules using an SPC 44 parameterization. In all systems, periodic boundary conditions were applied to all dimensions. As both the FAD cofactor and the acyl-CoA substrate are negatively charged (charge of −2 and −3, respectively), the system's total charge was neutralized by replacing an adequate number of water molecules with 1 (APO), 9 (FAD), or 25 (LIPID) sodium ions. Overall, the number of atoms in each system for pMCADwt was 214676 (APO), 214620 (FAD), and 214458 (LIPID), respectively.
The systems prepared for hMCADwt were solvated with 64182 (APO), 64079 (FAD), and 63941 (LIPID) SPC 44 water molecules. Again, these systems' total charge was neutralized with 4 (APO), 12 (FAD), and 28 (LIPID) sodium ions by replacing the same amount of water molecules. Overall, the number of atoms in each system for hMCADwt was 207910 (APO), 207869 (FAD), and 207731 (LIPID), respectively.
Molecular dynamics simulations
All simulations for both pMCADwt and hMCADwt proteins’ systems were performed using gromacs 4.6.3 38-41 package. GROMOS96 35, 36 53a6 37 force field and the simple point charge 44 (SPC) water model were used. After the systems’ preparation, and to remove clashes between the atoms, energy minimization runs were performed using the steepest descent algorithm. The minimization stopped when the maximum force acting on an atom was <1000 kJ/mol/nm.
Previously, all the systems were equilibrated under NVT ensemble simulations (100 ps) followed by 2 ns of an NPT ensemble simulations. The V-rescale weak coupling method 45 was used to heat the systems to 310 K and the Nosé-Hoover 46, 47 thermostat or Parrinello-Rahman 48 barostat was used to generate a rigorous NPT ensemble (T = 310 K, P = 1 bar, respectively). Positional restraints on heavy atoms were applied in NVT and NPT (1 ns) runs followed by an unrestrained NPT (1 ns) on heavy atoms of the FAD and substrates (FAD and LIPID systems). Bond lengths were constrained using LINCS 49, 50 and SETTLE 51 (water molecules) algorithms. A cut-off of 1.0 nm was used to compute the short-range electrostatic and van der Waals interactions. Long-range electrostatic interactions were calculated with the particle-mesh Ewald (PME) method 52, 53. Fully unrestrained production runs were performed over 100 ns under the same conditions, using the Nosé-Hoover 46, 47 thermostat/Parrinello-Rahman 48 barostat to control the temperature and the pressure, respectively.
Analysis of the MD trajectories
Several modules available in gromacs 4.6.3 38-41 package were used to analyze the MCAD structural stability parameters and its dynamic behavior for both porcine and human proteins. For each system, the root mean square deviation (RMSD) and the radius of gyration (Rg) of the Cα atoms were calculated by comparison with the initial crystallographic structures. The evolution of the secondary structure in function of time was calculated by the dssp 54, 55 software. To assess the protein flexibility, the root mean square fluctuation (RMSF) of the protein's residues was also calculated.
The volumes of the catalytic pockets of each protein system were estimated for all systems along the simulation using the voidoo 56 package.
To predict the binding sites for both FAD cofactor and fatty-acyl substrate, the amino acids lining the ligands within the catalytic pockets were primarily determined using the eposbp 57, 58 software and the relative polarities of the FAD and fatty-acyl substrate binding regions were calculated.
The ligands binding sites were also assessed by the g_contacts 59 module added to the gromacs program package. To estimate the relative free energies of binding (∆Gbind) for the FAD cofactor and the fatty-acyl substrate, the g_mmpbsa 60 module was used to numerically solve the Poisson–Boltzmann equation by linking GROMACS with APBS 61. The distances between cofactor, substrate, and protein's residues in the catalytic pockets, as well as the hydrogen bonds (HBs), were calculated using the g_dist, g_mindist, and g_hbond 62 modules, respectively. To study the catalytic pocket dynamics, the side-chain conformations of protein's residues in the binding pockets were also investigated for each system.
Visual inspections were performed with the vmd 1.9.1 63 and moe 2013.081 software.
Results and Discussion
Analysis of MCAD wild-type proteins stability
Root mean square deviation (RMSD) and radius of gyration (Rg)
The RMSD and Rg of the tetrameric (Figure 2) and monomeric forms (Figures S2 and S3) of both pMCADwt and hMCADwt proteins were plotted for each system (APO, FAD, and LIPID). Regarding the RMSD of the tetrameric forms, a similar behavior was observed in all simulations for both MCAD proteins, with the values increasing, respectively, in the first 15 ns (pMCADwt) and 20 ns (hMCADwt) of simulation time until a plateau was reached for both proteins. In all simulations after 60 ns, the MCAD proteins were considered stable (Figure 2 – left).
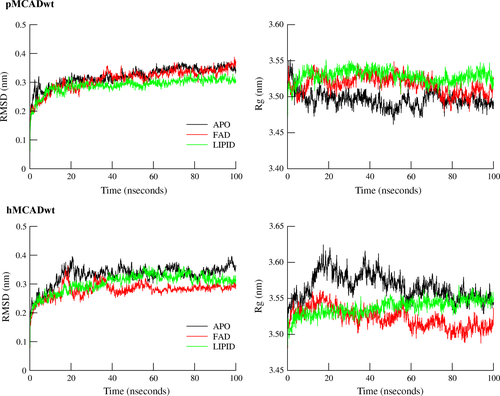
The mean RMSD values obtained from the different simulations revealed that both pMCADwt and hMCADwt tetrameric proteins were stable during the simulation, with respect to the corresponding starting crystallographic structures (PDB: 1UDY 4 and PDB: 1EGC 34- reversed) (Figure 2 – left). Nevertheless, a distinct behavior during the simulation time was found among monomers of pMCADwt protein in the absence of ligands (APO system) in contrast to a more homogeneous behavior found between monomers of the pMCADwt in the FAD and LIPID systems.
However, and unlike the pMCADwt, the RMSD values of individual monomers of the wt form of hMCAD protein revealed a similar behavior between them, for all the considered systems.
With respect to Rg, no significant differences between the crystallographic and the final MD structures were found among the systems tested in both pMCADwt and hMCADwt tetrameric proteins. This confirms that the proteins’ spatial organization of the final MD structures was maintained in the three systems and in both proteins (Figure 2 – right).
Nevertheless, the analysis of the protein′s behavior during the simulation time revealed an unstable profile in the absence of ligands (APO system), especially in the hMCADwt by contrast with the stable behavior found in the presence of the FAD and the fatty-acyl substrate (LIPID system) in both proteins.
Interestingly, the presence of only FAD (FAD system) within the hMCADwt structure seems to confer a slight stabilization effect to the protein along the simulation time.
Regarding the Rg values of each monomer of both wt proteins, while no major differences were observed among monomers of pMCADwt, for the APO, FAD, and LIPID systems, the monomers B and D in the hMCADwt displayed a more unstable behavior during the simulation time with respect to monomers A and C for all the three considered systems.
Protein secondary structure assessment
To validate the above-mentioned results, and to analyze the impact of the absence (APO system) or presence of ligands (FAD/LIPID systems) in the secondary structure of MCADwt proteins, the evolution of the secondary structure in function of time was also calculated applying the dssp 54, 55 software.
The results confirm that the secondary structure of both proteins remained stable in all simulations and comparable to the respective crystallographic structures (pMCADwt – 50 ± 2% α-helices and 14 ± 1% β-sheets; and hMCADwt – 53% α-helices and 14% β-sheets). These findings indicate that the absence of the FAD and the fatty-acyl substrate does not affect the MCAD secondary structure.
Root mean square fluctuation (RMSF)
Similar residue fluctuation profiles were observed in all systems and in both MCADwt proteins (Figure S4). The majority of the residues fluctuate below 2.0 Å and the largest fluctuations (above 4.0 Å) were detected in residues located in coils, α-helices exposed to solvent (residues 352–366) and β-sheets (residues 180–200).
The most stable residues (below 1.0 Å) are located in α-helices at the protein core, essential for the tetramer assembly and stability according to the β-factors of the crystallographic structures 4, 34.
Remarkably, we observe that some residues located in the catalytic pocket are equally stable (below 2.0 Å), especially those surrounding the FAD and the fatty-acyl substrate (residues 76–100 and 256–279).
Nonetheless, although both MCADwt proteins showed similar residue fluctuation profiles for all the three systems, the residue fluctuations seems to be more pronounced in monomers A and C of pMCADwt and in monomers A and D of hMCADwt demonstrating distinct behavior among monomers as already observed by the RMSD and Rg profiles during the MD simulations.
Characterization of the catalytic pockets
For the maximum catalytic efficiency of MCAD enzyme, the catalytic residue (E376) and the FAD cofactor must be accessible by the fatty-acyl substrate. The crystallographic structures of both MCADwt proteins showed that each catalytic pocket, where the E376 residue and the FAD are located, is formed by the two monomers of one dimer 4, 19. Modifications in the monomer's folding or assembly may compromise the formation of the catalytic pockets and impair MCAD function. It is therefore critical to throughly characterize the structure and dynamics of the catalytic pockets in the wt form of MCAD to further understand how mutations may affect its structure and ultimately the enzymatic function.
Catalytic pockets volume
One of the properties of the catalytic pockets that may change in the absence or presence of ligands is its internal volume. An estimate of the changes of this volume over the course of the simulation was performed with the voidoo 56 package. In addition, the spatial coordinates of the lining cavity residues and waters inside the catalytic pockets were also obtained.
The probability distribution of the mean volumes [P (V)], of the four binding pockets, in both proteins, was plotted for all systems tested (APO, FAD, and LIPID) (Figure 3) and for each monomer (Figures S5 and S6).
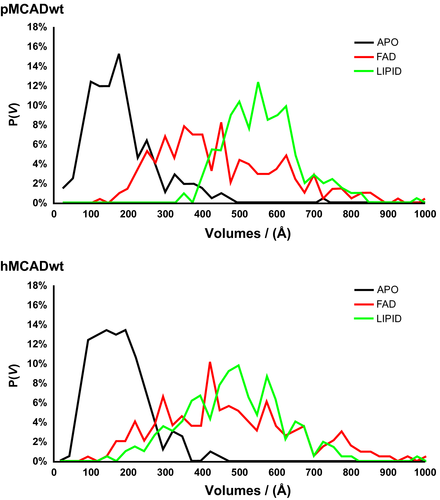
To correctly estimate the binding cavities volumes, the calculations were performed disregarding the FAD and the fatty-acyl substrate ligands.
The results obtained show that while in the absence of ligands (APO system) the catalytic pockets collapse and only residual volumes are observed, with the FAD cofactor alone (FAD system) or in the presence of both FAD and the fatty-acyl substrate (LIPID system) the formation of cavities with larger volumes is maintained in both MCAD proteins.
These findings indicate that the catalytic pockets are very dynamic and flexible and the absence of both FAD and fatty-acyl substrate leads to changes at the catalytic pocket architecture demonstrating that both ligands are important to delimit the volume of the catalytic pockets.
When analyzing the mean number of water molecules content inside the catalytic pockets of both proteins, no considerable differences were observed between the APO [pMCADwt (13) and hMCADwt (12)] and the FAD [pMCADwt (15) and hMCADwt (13)] systems. However, the number of water molecules within the catalytic pockets decreases in the presence of the fatty-acyl substrates (LIPID systems) [pMCADwt (7) and hMCADwt (6)], in agreement with the crystallographic data and previous studies in D-amino acid oxidase (DAOB) flavoprotein 64. Thus, the entry of the acyl-CoA molecule into the catalytic pockets is followed by a decrease in the total number of water molecules inside the cavities, to better accommodate the fatty-acyl substrate and provide a hydrophobic environment required for the α,β-dehydrogenation reaction 4, 5, 502
Cofactor and fatty-acyl substrate binding regions
As mentioned, each catalytic pocket is mainly formed by residues from both monomers of the dimer involving all three domains 4. The relative polarities showed that in both wt proteins, the FAD binding region is generally more hydrophilic, while the fatty-acyl substrate is mostly surrounded by hydrophobic residues [0.335 versus 0.225 (pMCADwt) and 0.302 versus 0.212 (hMCADwt), respectively].
As also defined in the crystallographic data, the FAD of one monomer has an extended conformation that enables the formation of HBs with residues from the neighboring monomer of the dimer, namely with R281, V350 (pMCADwt)/I350 (hMCADwt), G353, and N354, involving the oxygen atoms of the pyrophosphate and the adenine regions of the cofactor 3, 4. These HBs were also found in both MD structures of pMCADwt and hMCADwt proteins and their physical properties are depicted (Tables S1 and S2).
In general, and despite the HB lifetime (τ) decrease, an increase in the average number of HBs per timeframe (NHB) with more favorable energies of hydrogen bonding (ΔGHB) was observed between the FAD and the protein's residues, in the presence of fatty-acyl substrate (LIPID system). These findings suggest that the fatty-acyl substrate reinforces the HB network between the FAD of one monomer and the neighboring monomer contributing to increase the dimer cohesion. Additionally, HBs were observed between the FAD and a large number of residues lining the catalytic pockets of both MCAD proteins, being the most representative ones [>50% of contact frequencies over the course of the simulations; calculated by g_contacts 59] depicted in Figure 4.
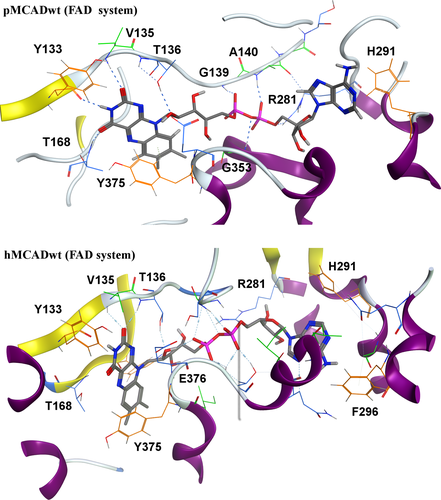
The FAD interaction pattern remains similar in the two FAD (Figure 4) and LIPID (Figure S7) systems, in agreement with the crystallographic data. The HB network seems to be distributed mainly in the pyrophosphate and flavin moieties of the FAD molecule in both MCAD proteins, being reinforced in the presence of the fatty-acyl substrate.
The direct analysis of the ligand interactions in both MCAD proteins and for both FAD (Figure 4) and LIPID (Figure S7) systems showed that the aromatic residues Y133 and Y375, near the flavin ring, as well as the H291 residue, surrounding the adenine group of the cofactor, seem to be important to stabilize the FAD cofactor inside the protein through additional non-bonded interactions, as seen by the high contact frequencies between these residues and the FAD cofactor during the simulations (Tables S3–S6).
While calculating the relative free energies of FAD binding [by g_mmpbsa 60] to the protein for all monomers, a similar behavior was found in both pMCADwt and hMCADwt proteins. In the absence of the fatty-acyl substrate inside the catalytic pockets (FAD system), the average binding energy for the FAD cofactor is −886 kJ/mol for pMCADwt and −845 kJ/mol for hMCADwt, decreasing in the presence of substrate (LIPID system) [pMCADwt (−1023 kJ/mol) and hMCADwt (−1016 kJ/mol)].
Thus, together with the data from the contact frequencies [calculated by g_contacts 59] and the analysis of the most prevalent HB [calculated by g_hbond 62] found between the cofactor and the protein, it is clear that the FAD binding improved as a result of the reinforcement of the FAD-protein non-bonded interactions induced by the fatty-acyl substrate binding.
As observed in Figure S8, and similar to the FAD cofactor, the fatty-acyl substrate is also anchored to protein by HBs with residues lining the catalytic pockets, mainly in the phosphate region of the substrate molecule in both MCAD proteins.
Furthermore, as mentioned above for the FAD interactions, the Y133 and Y375 aromatic residues also contribute to stabilize the fatty-acyl substrate inside the pocket by creating an aromatic cage that interacts with the terminal acyl group and the thioester region of the fatty-acyl substrate, respectively.
Interestingly, the aromatic residues F252 and F245 of both wt proteins interact with polar groups of the respective fatty-acyl substrates, suggesting an important role of these residues on the stability of the substrate binding to the protein (Tables S7 and S8).
Active site modifications
As mentioned, the molecular mechanism of MCAD enzymatic reaction has been extensively reported in previous studies 5, 25-31. In this section, we will focus in the active site dynamics, in the absence or presence of the fatty-acyl substrate.
The correct alignment between the flavin ring of FAD, the fatty-acyl moiety of the substrate, and the catalytic residue E376 in the MCAD enzyme is essential for the α, β-dehydrogenation reaction to occur 3, 4, 65. According to the crystallographic structure, we observed in both MCAD proteins that, upon complex formation, some residues changed their side-chain conformations to better accommodate the fatty-acyl substrate. The data gathered from MD simulations showed that in the presence of FAD (FAD system), the carboxylate group of E99, and the hydroxyl group of Y375 residues were able to interact by HBs (Tables S9 and S10). In the presence of the fatty-acyl substrate (LIPID system), the E99–Y375 HBs were broken allowing the fatty-acyl moiety of the substrate to bind deeper inside the pocket. These findings are in agreement with the crystallographic data, which emphasizes the alterations in the side-chain conformations of the E99 and Y375 residues as the most relevant fact to increase the pocket depth 5.
As also described in the crystallographic structure, and in other experimental studies 5, 51 in the LIPID systems, and for the two proteins, HBs were observed between the carbonyl oxygen of the fatty-acyl substrate with the 2′-OH of the ribityl group of FAD and the main-chain amide nitrogen of the catalytic residue (E376), (Tables S9 and S10). Nevertheless, the mean distance between the carbonyl oxygen of the fatty-acyl substrate with the 2′-OH of the ribityl group of FAD [pMCADwt (4.2 Å) and hMCADwt (8.9 Å)] and the main-chain amide nitrogen of E376 residue [pMCADwt (5.4 Å) and hMCADwt (9.8 Å)] may explain the reduced number of HBs per timeframe (NHB) detected in both proteins over the course of the simulations between these atom pairs.
As previously reported, the catalytic residue E376 plays an important role in the active site geometry and in the substrate chain length specificity of MCAD enzyme 19, 53. Unlike described in the crystallographic data, the simulations showed that in the LIPID system (not observed in FAD system), the carboxylate group (side chain) of the E376 residue switches dramatically toward the opposite side of the Cα-Cβ bond of the fatty-acyl substrate in both MCADwt proteins. The mean distance between the carboxyl oxygens of the E376 residue and the Cα atom of the fatty-acyl substrate [pMCADwt (7.2 Å) and hMCADwt (7.0 Å)] is not in agreement with the crystallographic data (4.2–4.6 Å) 4.
According to the crystallographic structure 34 of the double mutant E376G/T255E of the hMCAD enzyme, it was found an ‘active’ and a ‘resting’ conformations of its catalytic residue E255. The ‘active’ form corresponds to the correct orientation of the carboxylate group of E376 toward the Cα-Cβ bond of the fatty-acyl substrate, while in the ‘resting’ form its side chain is located at the opposite side of the substrate's Cα-Cβ bond.
Facing the above, a plausible hypothesis could be that during the simulated timescale (100 ns), the catalytic residue E376 adopts the ‘resting’ form in the presence of the fatty-acyl substrate (LIPID system), being stabilized by strong interactions with the R256 residue favoring the complex stabilization (Tables S9 and S10).
The R256 residue is conserved in the substrate binding domain of the acyl-CoA dehydrogenases family and according to previous studies 31, 66 plays an important role on MCAD catalysis. The simulations showed that in both pMCADwt and hMCADwt proteins, the mean distance between the carboxylate group of the E376 residue and the guanidine group (side chain) of the R256 residue was relatively similar in both APO [pMCADwt (5.4 Å) and hMCADwt (7.3 Å)] and FAD [pMCADwt (5.6 Å) and hMCADwt (5.9 Å)] systems, while in the LIPID system the mean distance between these residues is reduced [pMCADwt (4.0 Å) and hMCADwt (4.3 Å)], opposing to the available crystallographic data where this distance increases (4.8 Å) 4. Although these results do not fully agree with the crystallographic structure, they can be explained by a distinct orientation of the carboxylate group of the E376 residue toward the opposite side of the Cα-Cβ bond of the fatty-acyl substrate, observed in both wt proteins. In fact, HBs were observed between the R256 and E376 residues in all systems (Tables S9 and S10), being more frequent and stronger in the presence of the fatty-acyl substrate, due to side-chain rearrangements of both of these residues.
Conclusions
In this work, a comparison between two prepared and simulated models of MCADwt enzymes (porcine and human) was performed to provide insights into the dynamics behavior of the MCAD enzyme and validate these models to further MD calculations on MCAD variants. The structural stability of MCAD enzyme was firstly analyzed. From the comparison with the crystallographic structure, the obtained results suggest that both wt proteins are stable during the simulations, maintaining their spatial organization and the secondary structure; nevertheless, distinct behavior was found among monomers in both MCADwt proteins. The catalytic pockets dynamic revealed to be rather different from the crystallographic data in both wt proteins, more pronounced in the side-chain conformations of the catalytic residue E376 and R256 residue in the presence of the fatty-acyl substrate. Differences between the crystallographic and the MD structures have been also reported in other flavoproteins 67, 68.
From a comparative analysis between APO, FAD, and LIPID systems in both MCADwt proteins, it is possible to conclude that the FAD cofactor has an important structural role on the whole tetramer stability and dimer cohesion. In addition, the presence of the cofactor also prevents the catalytic pockets from collapsing, revealing to be important in the maintenance of the pockets’ volume.
The presence of the fatty-acyl substrate changes the dynamics of the catalytic pockets, especially the side-chain conformations of the residues located at active site, to better align the Cα-Cβ bond of the fatty-acyl substrate with the catalytic residue E376 and the flavin ring of FAD. The presence of the fatty-acyl substrate also leads to an increase in the FAD binding affinity, through the reinforcement of the non-bonded interactions between the FAD and the protein and a reduction in the number of water molecules inside the binding pockets.
Finally, the structural comparison between the two models of the porcine and human MCADwt proteins revealed that both enzymes are stable during the simulations. The quaternary structure stability and the subsequent analysis suggest that both proteins are essentially similar and that the reversion of the double mutant E376G/T255E of the hMCAD enzyme does not affect the structure of the protein neither its behavior. As such the crystallographic structure available for the pMCADwt and the reversed crystallographic structure of the double mutant E376G/T255E of the hMCAD enzyme may both be applied in further studies on MCAD variants and eventually for future high-throughput virtual screening toward the development of a pharmacological strategy to identify novel drugs for the treatment of MCAD disorder.
These computational data gathered bring a new complement to the currently ongoing experimental studies on the structural and functional characterization of MCAD variants identified in MCADD patients. Additionally, the MD model applied and the validated MCAD structure throughout this work is crucial for accelerating the discovery and development of compounds that may be potential stabilizers of MCAD variants and thus candidates for the treatment of MCAD deficiency.
Acknowledgments
The authors gratefully acknowledge Ricardo J. Ferreira M.Sc of the Department of Pharmaceutical Chemistry and Therapeutics, Natural Products Chemistry group, iMed.ULisboa, Faculty of Pharmacy, Universidade de Lisboa, for his valuable suggestions throughout the development of the studies herein presented. This work has been funded, in part, by iMed.ULisboa (UID/DTP/04138/2013) from Fundação para a Ciência e a Tecnologia (FCT), Portugal.




