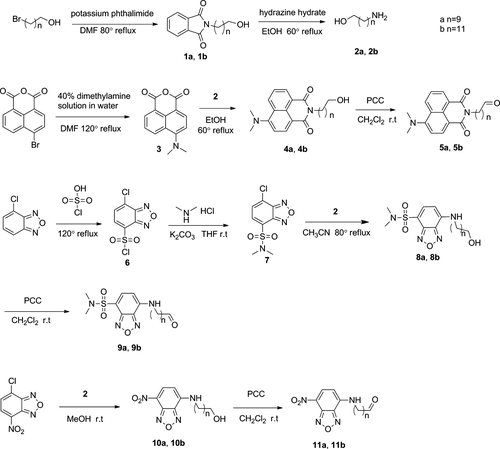
Discovery of New Substrates for LuxAB Bacterial Bioluminescence
Abstract
In this article, four novel substrates with long halftime have been designed and synthesized successfully for luxAB bacterial bioluminescence. After in vitro and in vivo biological evaluation, these molecules can emit obvious bioluminescence emission with known bacterial luciferase, thus indicating a new promising approach to developing the bacterial bioluminescent system.
Bioluminescence, as a particular form of chemiluminescence, is a natural phenomenon that emits visible light resulted from the reaction catalyzed by corresponding luciferase. Bioluminescence exists in most life-forms 1, for example, firefly, bacteria, fungi, and marine vertebrates and invertebrates. Bioluminescent technology has drawn more and more attention because of its wide application in the detection and imaging of biological process in vitro and in vivo 2, 3. Considering that the occurrence of bioluminescence does not demand an excitation light source as compared to fluorescence, this method has low background interference.
As the widespread prevalence in the marine environment, luminous bacteria have been isolated from multiple sources, including seawater, the light organs of marine luminous organisms and some symbiotic organisms of animals. So far, there are three major genera of luminous bacteria, including Vibrio, Photobacterium, and Photorhabdus (Xenorhabdus) 4-6. Luminescence is an especially striking activity controlled by gene regulation called quorum sensing in Vibrio 7, 8. Quorum sensing (QS) is a cell-to-cell communication that regulates gene expression and coordinates their behavior in line with the cell population density as a result of discerning molecules named autoinducers (AIs) 9. For example, the typical LuxR–LuxI quorum sensing system plays a significant role in the gene response of light production in Vibrio fischeri 10. The Vibrio bacterial bioluminescence reaction starts with oxidation of reduced flavin and a long-chain aliphatic (C10–C12) aldehyde that can be catalyzed by the corresponding luciferase. It should be underlined that the Vibrio bacterial bioluminescence is always bursting to reach the peak with a very fast speed. The concomitant maximum emission peaks of the bioluminescence reaction of various bacterial species are usually ranging from the wavelength of 472–500 nm. In this case, the aldehyde is regarded as a luciferin to some extent 11, 12.
It is known that the Vibrio bacterial luciferase is encoded by lux gene cassette that consists of a set of genes 13-15. The Vibrio bacterial luciferase protein is a heterodimer formed from the products of adjacent luxA and luxB genes 16. The fatty acid reductase multienzyme complex is encoded by the gene of luxC, luxD, and luxE, which catalyzes the synthesis of a long-chain aldehyde (the substrate for the luciferase reaction) from the fatty acid 4, 11. The product of luxG is a flavin reductase that regulates the regeneration of the reduced flavin mononucleotide in bioluminescence reaction 17.
The bacterial lux operon is an important bioreporter system that has been expanded for the detection of chemical signal molecules and imaging within a living host using cooled charge-coupled device (CCD) camera. Moreover, either full or parts of them (luxAB) could express in alternative host organisms such as Escherichia coli 13, 15, 18. After several decades of research, great contribution has been made to the development of modified lux gene cassette expressing in various prokaryotic cell lines even the eukaryotic cell line 19.
However, hitherto little work has been done in exploring the new substrates of the bacterial bioluminescence. Nowadays, the conventional substrates of bacterial bioluminescence are long-chain aliphatic aldehydes from natural source such as decanal, dodecanal, and tetradecanal 11. To fill this gap, herein we undertake an effort in designing a new series of compounds (5a, 5b, 9a, 9b, 11a, and 11b) with the property of fluorescence as the luciferin derivatives involved in the bacterial bioluminescence. Substituted 1,8-naphthalimide and 2,1,3-benzoxadiazole groups were chosen to be introduced in the structures of novel compounds. We want to probe and discuss the characteristics of bioluminescent and optical variation induced by introduction of fluorescent groups in order to obtain the better luciferin for bacterial bioluminescence.
Methods and Materials
Materials and instruments
All reagents and solvents available were used as received unless otherwise noted. All reactions were monitored by TLC with 0.25-mm silica gel plates (60GF-254). UV light, iodine stain, and ninhydrin were used to visualize the spots. Silica gel was utilized for column chromatography purification. 1H NMR and 13C-NMR were recorded on a Bruker DRX spectrometer at 300 or 400 MHz, δ in parts per million and J in hertz, using TMS as an internal standard. ESI-MS spectra were recorded on an API 4000 spectrometer (AppliedBiosystems, Foster city, CA, USA). Melting points were determined uncorrected on an electrothermal melting point apparatus. HPLC tests were performed with Agilent Technologies 1260 liquid chromatography (Singapore City, Singapore). Water used for the fluorescence and bioluminescence studies was doubly distilled and further purified with a Mill-Q filtration system (Millipore, Watertown, MA, USA). Bioluminescence measurements were determined with an IVIS Kinetic (Caliper Life Sciences, Hopkinton, MA, USA) equipped with a cooled charge-coupled device (CCD) camera for bioluminescent imaging. Bioluminescence spectra were measured with F-2500 FL Spectrophotometer (HITACHI High technologies Corporation, Tokyo, Japan). Decanal used in bioluminescence assay was purchased from Sigma (Shanghai, China).
Organic synthesis of novel substrates
We have synthesized six novel compounds as described in Scheme 1.
- To a stirred solution of 10-bromodecanol (800 mg, 3.37 mmol) in dimethylformamide (DMF) was added potassium naphthalimide (687 mg, 3.71 mmol). The reaction mixture was refluxed at 80 °C for 2 h and then cooled to the room temperature. After that, water was added to the mixture, and precipitation of crude product was collected by filtration and washed with water. The product was recrystallized from a mixture of dichloromethane and petroleum ether and then was dried in vacuo to afford 1 g of pure product (1a) as a pale powder. Yield: 98%. Mp 62–64 °C. 1H NMR (400 MHz, CDCl3): δ 7.84 (m, 2H), 7.71 (m, 2H), 3.65 (m, 4H), 1.67 (m, 2H), 1.55 (m, 2H), 1.33˜1.28 (m, 12H). ESI-MS: m/z 304.5 [M + H]+, 321.5 [M + NH4]+, 326.5 [M + Na]+.
- 2-(12-hydroxydodecyl)isoindoline-1,3-dione (1b): pale powder; yield 98%. Mp 63–65 °C. 1H NMR (400 MHz, CDCl3): δ 7.84 (m, 2H), 7.71 (m, 2H), 3.66 (m, 4H), 1.67 (m, 2H), 1.56 (m, 2H), 1.32˜1.26 (m, 16H). ESI-MS: m/z 332.7 [M + H]+, 349.6 [M + NH4]+, 354.5 [M + Na]+.
- To a stirred solution of 1a (330 mg, 0.99 mmol) in ethanol (10 mL) was added hydrazine hydrate (85%, 220 mg). The reaction mixture was refluxed at 80 °C for 2.5 h, resulting in the formation of a white precipitate. The suspension was cooled and concentrated. The white solid was triturated with dichloromethane with sonication and filtered. The trituration/sonication/filtration sequence was repeated. Then, the combined filtrates were concentrated to provide 130 mg of pale powder (2a). Yield: 69%. Mp 67–69 °C. 1H NMR (400 MHz, CDCl3) : δ 3.57 (t, J = 6.8 Hz, 2H), 2.62 (t, J = 7.2 Hz, 2H), 1.46˜1.52 (m, 2H), 1.37 (m, 2H), 1.27˜1.22 (m, 12H). MS: m/z 174.4 [M + H]+.
- 12-amino-1-dodecanol (2b): pale powder; yield 55%; Mp 66–68 °C. 1H NMR (300 MHz, CDCl3) : δ 3.63 (t, J = 6.6 Hz, 2H), 2.68 (t, J = 6.9 Hz, 2H), 1.59˜1.52 (m, 4H), 1.46˜1.39 (m, 2H), 1.28 (m, 14H). MS: m/z 202.4 [M + H]+.
- A total of 9.76 g of dimethylamine aqueous solution (40%) and a catalytic amount of CuSO4 (270 mg) were added to a suspension of 4-bromonaphthalene-1,8-dicarboximide (3 g, 10 mmol) in DMF (30 mL). The mixture was then refluxed at 160 °C for 2 h, after which the solvent was evaporated under vacuum. The product 3 was crystallized from ethanol, dichloromethane, and petroleum ether as a yellow solid 20. Yield: 52%. Mp 205–207 °C. 1H NMR (400 MHz, CDCl3) δ 8.49 (dd, J = 7.2 Hz, 1.2 Hz, 1H), 8.41 (dd, J = 8.8 Hz, 1.2 Hz, 1H), 8.38 (d, J = 8.4 Hz, 1H), 7.61 (m, 1H), 7.03 (m, 1H), 3.11 (s, 6H). MS: m/z 242.4 [M + H]+, 259.3 [M + NH4]+, 264.3 [M + Na]+.
- A solution of 3 (100 mg, 414 μmol) in EtOH (3 mL) was added to a solution of 2a (87 mg, 497 mmol) in EtOH (10 mL). The reaction was stirred and refluxed at 80 °C for 3 h. Then, the mixture was cooled to room temperature and concentrated under reduced pressure. The crude product was subjected to chromatography on silica gel (PE/EtOAC 5:1–3:1) to give yellow viscous oil. Yield: 69%. 1H NMR (400 MHz, CD3Cl) δ 8.59 (dd, J = 7.4 Hz, 1H), 8.50 (d, J = 8 Hz, 1H), 8.46 (dd, J = 8.6 Hz, 1H), 7.68 (m, 1H), 7.14 (d, J = 8.4 Hz, 1H), 4.17 (t, J = 7.6 Hz, 2H), 3.66 (m, 2H), 3.12 (s, 6H), 1.74 (m, 2H), 1.58 (m, 2H), 1.44 (m, 2H), 1.35˜1.27 (m, 12H). ESI-MS m/z: 397.5 [M + H]+.
- 4-N,N-(dimethyl)aminonaphthalene-9-N-(12-hydroxydodecyl)-1,8-dicarboximide (4b): yellow viscous oil. Yield: 76%. 1H NMR (400 MHz (CD3)2OD) δ 8.43 (dd, J = 8.4 Hz, 1H), 8.38 (dd, J = 7.2 Hz, 1.2 Hz, 1H), 8.28 (dd, J = 8.4 Hz, 1.2 Hz, 1H), 7.63 (m, 1H), 7.13 (m, 1H), 3.98 (t, J = 7.4 Hz, 2H), 3.38 (t, J = 6.6 Hz, 2H), 3.00 (s, 6H), 1.57 (m, 2H), 1.35 (m, 2H), 1.25 (m, 2H), 1.16 (m, 12H). ESI-MS m/z: 425.6 [M + H]+, 447.6 [M + Na]+.
- To a suspension of compound 4a (80 mg, 201 μmol) and silica gel(160 mg) in dry dichloromethane was added pyridinium chlorochromate complex (60 mg, 282 μmol) with stirring at 0 °C. Then the reaction mixture was stirred at room temperature under Ar atmosphere and protected from light overnight. The mixture was concentrated under reduced pressure and then was subjected to chromatography on silica gel (PE/EtOAC 10:1) to give very viscous yellow viscous oil. Yield: 44%. Analytical RP HPLC (Phenomenex, C8, 250 × 4.6 mm column): methanol:water 75:25, 1.5 mL/min at 440 nm, Rt: 11.177 min, 100%. 1H NMR (400 MHz, CDCl3) δ 9.68 (s, 1H), 8.49 (dd, J = 7.4 Hz, 1 Hz, 1H), 8.41 (d, J = 8.4 Hz, 1H), 8.36 (dd, J = 8.4 Hz, 0.8 Hz, 1H), 7.59 (m, 1H), 7.04 (d, J = 8.4 Hz, 1H), 4.08 (t, J = 7.6 Hz, 2H), 3.03 (s, 6H), 2.33 (td, J = 7.4 Hz, 1.9 Hz, 2H), 1.65 (m, 2H), 1.54˜1.47 (m, 2H), 1.36˜1.32 (m, 2H), 1.29˜1.23 (m, 8H). 13CNMR (100 MHz, CDCl3) δ 201.9, 163.6, 163.1, 155.9, 131.6, 130.1, 129.9, 129.2, 124.3, 123.9, 122.2, 114.2, 112.3, 43.8, 43.9, 39.2, 28.3, 28.3, 28.1, 27.1, 26.1. HR-MS: m/z calcd for [C24H30N2O3 + H]+ 395.2335, found: 395.2323 [M + H]+.
- 4-N,N-(dimethyl)aminonaphthalene-9-N-(11-aldehyde-dodecyl)-1,8-dicarboximide (5b): very viscous yellow viscous oil; yield: 46%. Analytical RP HPLC (Phenomenex, C8, 250 × 4.6 mm column): methanol:water 80:20, 1.5 mL/min at 440 nm, Rt: 10.654 min, 100%. 1H NMR (400 MHz, CDCl3) : δ 9.76 (s, 1H), 8.57 (d, J = 7.2 Hz, 1H), 8.48 (d, J = 8.4 Hz, 1H), 8.44 (d, J = 8.4 Hz, 1H), 7.67 (t, J = 7.8 Hz, 1H), 7.13 (d, J = 8.4 Hz, 1H), 4.15 (t, J = 7.6 Hz, 2H), 3.11 (s, 6H), 2.42 (td, J = 7.3 Hz, 1.7 Hz, 2H), 1.73˜1.68 (m, 2H), 1.64˜1.60 (m, 2H), 1.43˜1.26 (m, 14H). 13CNMR (100 MHz, CDCl3) δ 203.0, 164.6, 164.1, 156.9, 132.6, 131.1, 131.0, 130.2, 125.4, 124.9, 123.2, 115.2, 113.4, 44.8, 43.9, 40.3, 29.7, 29.5, 29.4, 29.3, 29.2, 28.2, 27.2, 22.1. HR-MS: m/z calcd for [C26H34N2O3 + H]+: 423.2648, found: 423.2640 [M + H]+.
- A total of 4 mL of chlorosulfonic acid was added dropwise to 4-chloro-2,1,3-benzoxadiazole (0.6 g, 3.88 mmol) in a round bottom flask at 0. After completion, the reaction mixture was heated at 120 °C for 8 h under Ar atmosphere. The reaction mixture was dropwise poured into ice water, and the precipitation of crude product was collected by filtration and washed with water. The product 6 (0.59 g) was crystallized from dichloromethane and petroleum ether as a light brown crystal. Compound 6 was used in the next step without further purification 21. Yield: 60%. 1H NMR (400 MHz, CDCl3): δ 8.21 (d, J = 8 Hz, 1H), 7.68 (d, J = 8 Hz, 1H). ESI-MS: m/z 233.1 [M–Cl + OH]−.
- To a bottom flask containing 10 mL dried anhydrous THF, dimethylamine hydrochloride (116 mg, 1.42 mmol) and K2CO3 (197 mg, 1.42 mmol) were dissolved and stirred at room temperature for 30 min. Then, the solution above was added dropwise to the THF solution of compound 6 with stirring at 0 °C. After the addition, the solution was further stirred for 6 h at room temperature. After removal of solvent in vacuo, the residue was purified by silica gel column chromatography (PE/EtOAC 5:1) to afford compound 7 (190 mg) as a white powder 22. Yield: 61%. 1H NMR (400 MHz, CDCl3): δ 7.97 (d, J = 7.6 Hz, 1H), 7.56 (d, J = 7.2 Hz, 1H), 2.97 (s, 6H). ESI-MS: m/z 262.3 [M + H]+, 279.3 [M + NH4]+, 284.3 [M + Na]+.
- To a solution of compound 2a (60 mg, 347 μmol) in acetonitrile with stirring, was added dropwise the solution of compound 7 (70 mg, 267 μmol). Then, the mixture was refluxed at 60 °C for 8 h. After removing the solvent in vacuo, the residue was purified by silica gel column chromatography (PE/EtOAC 5:2) to afford compound 8a as a yellow powder (59 mg, 55%). Mp 83–85 °C. 1H NMR (400 MHz, CDCl3) : δ 7.83 (d, J = 8 Hz, 1H), 6.05 (d, J = 8 Hz, 1H), 5.57 (t, J = 5 Hz, 1H), 3.57 (m, 2H), 3.32 (m, 2H), 2.80 (s, 6H), 1.74˜1.66 (m, 2H), 1.52˜1.46 (m, 2H), 1.41˜1.35 (m, 2H), 1.29˜1.25 (m, 10H). ESI-MS: m/z 399.4 [M + H]+, 416.5 [M + NH4]+, 421.4 [M + Na]+.
- 4-N-(12-hydroxydodecyl)-7-N,N-dimethylsulfonic-2,1,3-benzoxadiazole (8b): yellow powder; yield: 50%. Mp 73–75 °C. 1H NMR (400 MHz, CDCl3) : δ 7.83 (d, J = 8 Hz, 1H), 6.05 (d, J = 8 Hz, 1H), 5.57 (t, J = 5.4 Hz, 1H), 3.56 (s, 2H), 3.31 (m, 2H), 2.80 (s, 6H), 1.72˜1.66 (m, 2H), 1.51˜1.46 (m, 2H), 1.40˜1.35 (m, 2H), 1.27˜1.22 (m, 14H). ESI-MS: m/z 427.5 [M + H]+, 444.7 [M + NH4]+, 449.5 [M + Na]+.
- To a suspension of compound 8a (45 mg, 113 μmol) and silica gel (100 mg) in dry dichloromethane was added pyridinium chlorochromate complex (36 mg, 167 μmol) with stirring at 0 °C. Then, the reaction mixture was stirred at room temperature under Ar atmosphere and protected from light overnight. The mixture was concentrated under reduced pressure and then was subjected to chromatography on silica gel (PE/EtOAC 5:1) to give yellow powder (25 mg, 32%). Analytical RP HPLC (Phenomenex, C8, 250 × 4.6 mm column): methanol:water 65:35, 1.5 mL/min at 440 nm, Rt: 10.098 min, 99%. Mp 80–82 °C. 1H NMR (400 MHz, CDCl3) δ 9.79 (s, 1H), 7.92(d, J = 8 Hz, 1H), 6.14 (d, J = 8 Hz, 1H), 5.64 (t, J = 5 Hz, 1H), 3.40 (m, 2H), 2.89 (s, 6H), 2.46 (td, J = 7.2 Hz, 1.8 Hz, 2H), 1.81˜1.75 (m, 2H), 1.67˜1.64 (m, 2H), 1.48˜1.44 (m, 2H), 1.41˜1.35 (m, 8H). 13CNMR (75 MHz, CDCl3) δ 202.8, 146.3, 144.4, 140.7, 139.6, 109.4, 98.7, 43.9, 43.6, 37.8, 29.2, 29.2, 29.1, 28.6, 27.0, 22.0. HR-MS: m/z calcd for [C18H28N4O4S + H]+: 397.1910, found: 397.1901 [M + H]+.
- 4-N-(11-aldehyde-dodecyl)-7-N,N-dimethylsulfonic-2,1,3-benzoxadiazole (9b): yellow powder; yield: 43%. Analytical RP HPLC (Phenomenex, C8, 250 × 4.6 mm column): methanol:water 70:30, 1.5 mL/min at 440 nm, Rt: 11.663 min, 99%. Mp 78–79 °C. 1H NMR (400 MHz, CDCl3) δ 9.79 (t, J = 1.8 Hz, 1H), 7.92 (d, J = 8 Hz, 1H), 6.14 (d, J = 8 Hz, 1H), 5.65 (t, J = 4.8 Hz, 1H), 3.40 (m, 2H), 2.89 (s, 6H), 2.45 (td, J = 7.3 Hz, 1.7 Hz, 2H), 1.82˜1.76 (m, 2H), 1.69˜1.62 (m, 2H), 1.49˜1.44 (m, 2H), 1.38˜1.32 (m, 12H). 13CNMR (100 MHz, CDCl3) δ 202.8, 146.3, 144.5, 140.7, 139.6, 109.5, 98.7, 43.9, 43.6, 37.8, 29.4, 29.3, 29.3, 29.2, 29.1, 28.6, 27.0, 22.1. HR-MS: m/z calcd for [C20H32N4O4S + H]+: 425.2223, found: 425.2212 [M + H]+.
- To a stirred solution of compound 2a (24 mg, 140 μmol) in methanol was added dropwise the solution of 4-chloro-7-nitro-1,2,3-benzoxadiazole (28 mg, 140 μmol). Then, the mixture was further stirred at room temperature overnight. After removing the solvent in vacuo, the residue was purified by silica gel column chromatography (PE/EtOAC 10:3) to afford compound 10a as an orange powder (30 mg, 64%). Mp 104–105 °C. 1H NMR (400 MHz, CDCl3) δ 8.51 (d, J = 8.4 Hz, 1H), 6.17 (d, J = 8.4 Hz, 1H), 3.65 (t, J = 6.6 Hz, 2H), 3.48 (m, 2H), 1.81 (m, 2H), 1.59 (m, 2H), 1.47 (m, 2H), 1.37 (m, 8H), 1.25 (m, 2H). ESI-MS: m/z 337.6 [M + H]+, 354.5 [M + NH4]+, 359.6 [M + Na]+.
- 4-N-(12-hydroxydecyl)-7-nitro-2,1,3-benzoxadiazole (10b):orange powder; yield: 45%; Mp 108–109 °C. 1H NMR (400 MHz, CDCl3) δ 8.50 (d, J = 8.4 Hz, 1H), 6.24 (s, 1H), 6.17 (d, J = 8.4 Hz, 1H), 3.65 (t, J = 6.6 Hz, 2H), 3.49 (m, 2H), 1.81 (m, 2H), 1.47 (m, 2H), 1.29 (m, 16H). ESI-MS: m/z 365.5 [M + H]+, 382.6 [M + NH4]+, 387.5 [M + Na]+.
- To a suspension of compound 10a (50 mg, 149 μmol) and silica gel (100 mg) in dry dichloromethane was added pyridinium chlorochromate complex (45 mg, 208 μmol) with stirring at 0 °C. Then, the reaction mixture was stirred at room temperature under Ar atmosphere and protected from light overnight. The mixture was concentrated under reduced pressure and then was subjected to chromatography on silica gel (PE/EtOAC 10:1) to give orange powder (20 mg, 41%). Analytical RP HPLC (Phenomenex, C8, 250 × 4.6 mm column): methanol:water 60:40, 1.0 mL/min at 440 nm, Rt: 24.400 min, 99%. Mp: 91–92 °C. 1H NMR (300 MHz, CDCl3) δ 9.77(s, 1H), 8.48 (d, J = 8.7 Hz, 1H), 6.31 (s, 1H), 6.18 (d, J = 8.7 Hz, 1H), 3.50 (m, 2H), 2.44 (td, J = 7.2 Hz, 1.2 Hz, 2H), 1.82 (m, 2H), 1.64 (m, 2H), 1.46 (m, 2H), 1.33 (m, 8H). 13CNMR (100 MHz, CDCl3) δ 202.8, 144.3, 143.9, 143.9, 136.5, 124.0, 98.5, 44.0, 43.9, 29.2, 29.1, 29.0, 28.5, 26.9, 22.0. HR-MS: m/z calcd for [C16H22N4O4 + H]+: 335.1719, found: 335.1717 [M + H]+.
- 4-N-(11-aldehyde-decyl)-7-nitro-2,1,3-benzoxadiazole (11b): orange powder; yield: 51%; Analytical RP HPLC (Phenomenex, C8, 250 × 4.6 mm column): methanol:water 60:40, 1.0 mL/min at 440 nm, Rt: 21.591 min, 99%. Mp: 98–99 °C. 1H NMR (300 MHz, CDCl3) δ 9.77 (t, J = 1.6 Hz, 1H), 8.49 (d, J = 8.7 Hz, 1H), 6.32 (s, 1H), 6.18 (d, J = 8.4 Hz, 1H), 3.50 (m, 2H), 2.43 (td, J = 7.2 Hz, 1.7 Hz, 2H), 1.82 (m, 2H), 1.61 (m, 2H), 1.48˜1.40 (m, 2H), 1.30 (m, 12H). 13CNMR (100 MHz, CDCl3) δ 202.9, 144.3, 143.9, 143.9, 136.4, 124.1, 98.5, 44.0, 43.9, 29.7, 29.4, 29.3, 29.2, 29.1, 28.6, 26.9, 22.0. HR-MS: m/z calcd for [C18H26N4O4 + H]+: 363.2032, found: 363.2026 [M + H]+.
Compounds 5a, 5b, 9a, 9b, 11a, and 11b should be kept with N2 at −20 °C for a long-time store.
Fluorescence spectra measurement
A 95% ethanol stock solution of the respective new substrate (10 mm) was diluted to the corresponding concentration with PBS (50 mm, pH 7.0). The PBS solution of the compound (200 μm, 150 μL) was added to a 96-well black flat-bottom microscale plate and then scanned with the Thermo Scientific Varioskan Flash microplate reader (Thermo Electron Corporation, Waltham, MA, USA).
Construction of the plasmid pCS26-luxAB
The 5935-bp fragment was obtained by PCR using primers luxABF and luxABR from pK316 23, and then, the fragment was self-ligated using the Gibson assembly method 24 to generate the plasmid pCS26-luxAB, in which luxAB is under the control of kasOp* promoter (Figure S2).
- luxABF: agagtgaggaaccctcgaggggatcctctagttgcg.
- luxABF: agagtgaggaaccctcgaggggatcctctagttgcg.
Bioluminescence assay
To determine the bioluminescence properties of the novel derivatives, plasmids pCS26-luxAB encoding bacterial luciferase (luxAB) were transfected into E. coli cultured in the plate. Specifically, E. coli was streak-seeded on fresh LB plates and then cultured in the presence of 25 μg/mL kanamycin. Colonies appeared after overnight incubation at 37 °C. A single colony was picked from the LB plate. Then, this strain was grown overnight with aeration (250 rpm) at 37°C in 5 mL of LB medium with antibiotics (20 μg/mL kanamycin) to OD600 1.2–1.4. The E. coli cultures were washed twice with fresh M9 medium, and then, the cell pellet was resuspended in fresh M9 medium and diluted to OD600 1.0. The E. coli culture was prepared for each measurement.
All compounds were freshly dissolved in and diluted to appropriate concentrations in PBS (50 mm, pH 7.0) with a little addition of 95% EtOH (final ethanol concentration <0.5% v/v) for each measurement. In all the measurements, the final ethanol concentration in the sample solution was kept constant at 0.5% (v/v) to avoid the effect of ethanol on the BL reaction. To measure bioluminescence intensity, the solution of compound (40 μL) was added to a 96-well black flat-bottom microscale plate and then the fresh E. coli cultures (80 μL) was added and mixed quickly. Bioluminescence intensities of native decanal and the derivatives were immediately measured with an IVIS Kinetic (Caliper Life Sciences) equipped with a cooled charge-coupled device (CCD) camera with the exposure time of 60 seconds. The measurement would last 20–30 min to determine the kinetic profiles of bioluminescence at different concentrations.
For the recording of bioluminescence spectra and relative quantum yields (RQYs), an aliquot of the E. coli culture (300 μL) with OD600 1.0 was mixed with PBS (150 μL) containing decanal (0.5 mm) or derivative (1 mm) in a quartz cell and the mixture was immediately measured with a F-1200 fluorescence spectrophotometer in luminescence mode with the lamp off at a scan rate of 3000 nm/min. The wavelengths of maximal bioluminescence intensities (λmax) and the quantitative bioluminescence spectra were determined using the instrument software (fl solutions ver. 2.1). The quantum yield (QY) was calculated by dividing the measured total number of photons by the number of substrate molecules in the solution 25, 26.
In vivo Imaging
Novel substrates 5a and 5b were chosen for bioluminescence imaging in mice. All compounds were freshly dissolved in and diluted to appropriate concentrations in 0.9% NaCl with a little addition of 95% EtOH (final ethanol concentration <0.5% v/v) for this measurement. The fresh E. coli culture was prepared as the same way of bioluminescence measurement. To demonstrate this functionality, 8-week-old female nude mice (weight: 18 ± 2 g) were treated with anesthetic agent isoflurane and then were subcutaneously injected with solution of compound (50 μL) and then were subcutaneously injected with E. coli culture (100 μL) in situ quickly. The bioluminescence was detected at once with an exposure time of 60 seconds. The next injection area of back skin would be changed after a finish of a set of subcutaneously injection.
Results and Discussion
Bioluminescence properties
Four compounds (5a, 5b, 9a, and 9b) have the capacity to emit a significant bioluminescence light in the presence of bacterial luciferase of Photorhabdus luminescens. However, compounds 11a and 11b exhibited no response to the bacterial luciferase. Therefore, we only discussed the bioluminescent characteristics of 5a, 5b, 9a, and 9b in this article. In addition, all compounds have fluorescence properties with the maximum excitation around 460 nm and the maximum emission in the range of 547–575 nm (Figure S1).
The recognition-dependent bioluminescence properties of four new compounds were well investigated using E. coli that transfected with the plasmid carrying the gene luxAB of Photorhabdus luminescens, considering that the luciferase protein derived from the lux gene of Photorhabdus luminescens is more thermostable than of Vibrio harveyi and Vibrio fischeri 19.
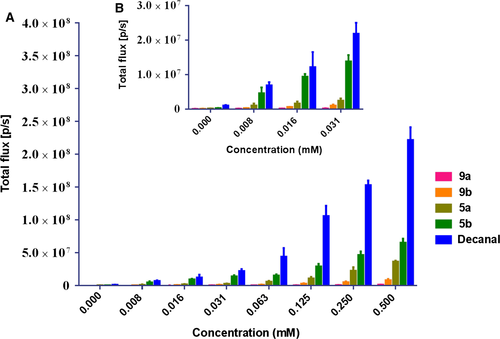
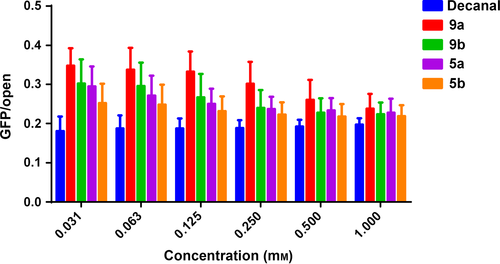
Four of our novel compounds displayed significant bioluminescence properties with bacterial luciferase. A comparison of the bioluminescent intensities of the new molecules and decanal is depicted in Figure 1. The bioluminescence intensities of them all were weaker than that of the native decanal. However, compounds 5a and 5b show better intensities among them. What's more, compound 5b displays similar intensity compared with decanal when they both were in relatively low concentrations (Figure 1B). Therefore, 5b has an advantage over them in the emission of bacterial bioluminescence. The 5a and 5b were more excellent than the 9a and 9b, and this may be due to their better aqueous solubility and high polarity.
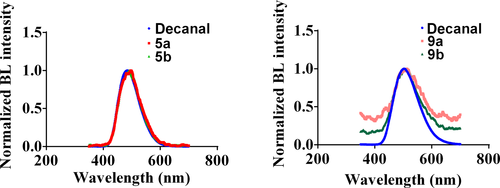
The comparison of the bioluminescent spectra of four new compounds and native decanal is depicted in Figure 2. The emission wavelengths of four new derivatives and native decanal are very similar. They just show tiny redshifted emission compared to that of decanal.
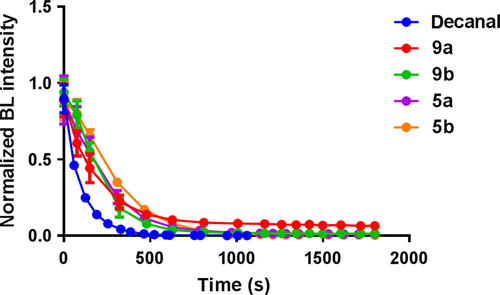
Moreover, we also have focused on the kinetic characteristics of our novel substrates. We evaluated the intensity–time-dependent bioluminescence (Figure 4). The bioluminescence decay halftime was calculated as shown in Table 1. We found that our compounds showed at least twofold longer halftimes than decanal. The best one (5b) displayed approximately threefold longer decay halftime than decanal. As we know, the bacterial bioluminescence is always bursting to reach the peak with a very fast speed and then is quenched soon. It is obvious that the bioluminescence induced by all of our compounds could decrease much slowly, which means that the persistence of our compounds is superior.
| Compounds | Decanal | 9a | 9b | 5a | 5b |
| BL half time (seconds)a | 66.62 | 132.7 | 149.2 | 169.0 | 221.8 |
| Kmb (mm) | 0.11 ± 0.02 | 1.79 ± 0.42 | 0.72 ± 0.09 | 0.72 ± 0.10 | 0.26 ± 0.03 |
| Vmaxb (s/p) | (8.42 ± 0.33) × 108 | (7.65 ± 1.07) × 106 | (2.35 ± 0.11) × 107 | (9.88 ± 0.51) × 107 | (1.07 ± 0.03) × 108 |
| RQYsc | 1 | 0.014 | 0.026 | 0.036 | 0.043 |
- a BL halftime was calculated using graphpad prism software and taken advantage of the values when compounds were 0.25 mm.
- b Michaelis constant Km and maximum rate Vmax were estimated with the Michaelis–Menten kinetics equation using graphpad prism software. The values are shown by means ± SD of three independent assays performed in triplicate.
- c Relative quantum yields were obtained with a F-1200 fluorescence spectrophotometer in luminescence mode with the lamp off. The quantitative bioluminescence spectra were calculated by the instrument software fl solutions ver. 2.1. The concentration of decanal was 0.5 mm and others were 1 mm.
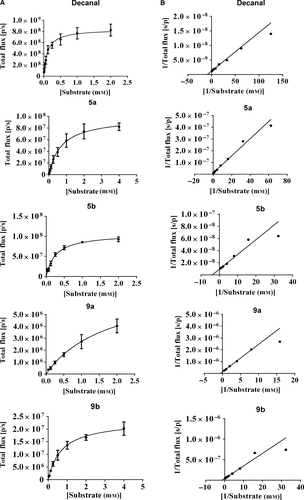
Furthermore, all of these molecules were investigated for their kinetic characteristics. By the Lineweaver–Burk plot, we have obtained the Michaelis–Menten parameters Km and Vmax to evaluate their combination mode with luciferase (Table 1 and Figure 5). Compound 9a was the worst with a high Km and a low Vmax value. Compounds 9b and 5a showed moderate Km values. The Km of compound 5b was near that of decanal. Further, the Vmax values of 5b and 5a were similar and were basically in the same magnitude of decanal. In a word, compound 5b showed the best kinetic characteristics. It is proved that our compounds could combine with the luciferase slowly so as to induce the bioluminescence reaction lasting longer with the low concentration.
As we all know, the quantum yield is one of the important properties of bioluminescence substrates. The relative quantum yields of all novel substrates were calculated and are exhibited in Table 1. As we can see, compound 5b behaved best and compound 5a took the second place among the new substrates. However, the RQY values of them were much lower than that of decanal in fact.
In vivo imaging
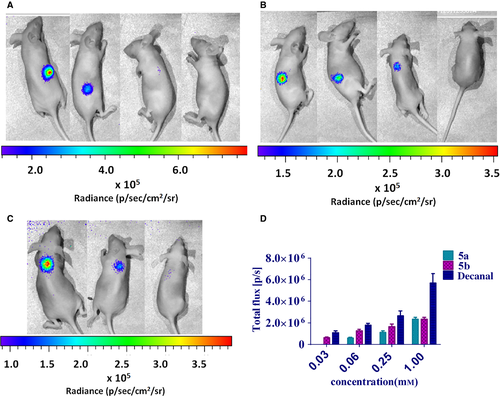
The LuxAB system has been previously used in whole animal bioluminescent imaging 16, 18, 19. The new substrates 5a and 5b could also be applied to bioluminescent imaging in vivo (Figure 6). The results of bioluminescence imaging in whole animal indicated that 5a and 5b displayed similar activities when they were confronted with decanal at the moderate concentrations in in vivo imaging. It is the first step to explore the new substrates that could be used in in vivo imaging. Because of the longer halftime of our compounds, they might have the potential to image in vivo for a long-lasting test.
The chemical structures of novel substrates were modified with fluorescent groups, which could make them possess optical properties. The modification with these polar groups also resulted in the change of physical state. The novel substrates were solids so as to be easier to carry and keep. In comparison with decanal, their polarity and water solubility had an improvement result from the structural modification, which might promote the interaction with luciferase. When it comes to structure–activity relationship in detail, compounds 5a and 5b exhibited better bioluminescence intensities and kinetic characteristics than 9a and 9b, which indicated that the introduction of 1,8-naphthalimide group was more appropriate compared with 2,1,3-benzoxadiazole group. In addition, 5b seemed always to exceed 5a, and 9b was always better than 9a. It is suggest that 12C aliphatic chain length was more suitable to combine with the luciferase in the bioluminescence reaction. Compounds 5a and 5b had the potential to be used in the animal bioluminescent imaging because their performance approached that of decanal in certain circumstances. In general, all four compounds could act as the substrates of bacterial bioluminescence although they did not surpass the conventional substrate in all aspects.
Conclusion
In this work, four types of novel bacterial bioluminescence derivatives with fluorescent property have been successfully developed. All of them showed apparent bioluminescence emission in combination with E. coli that transfected with a plasmid carrying luxAB. The bioluminescence induced by our compounds is less bright than that by decanal. The brightest emission of our compounds was observed in the case of the compound 5b. Interestingly, they all showed much longer halftime in comparison with decanal, which suggests that our substrates could emit bioluminescence steadily and continuously.
It has been demonstrated for the first time that a structural modification of the long-chain aliphatic aldehyde can result in bacterial bioluminescence substrates with obvious bioluminescence emission. We believe that the knowledge gained through this study is of significance for the continued elucidation of the bacterial bioluminescence reaction and contributes to the development of novel bacterial bioluminescence substrates with superior optical properties.
Acknowledgments
This work was supported by grants from the National Program on Key Basic Research Project (no. 2013CB734000), the National Natural Science Foundation of China (no. 81370085), and the Independent Innovation Foundation of Shandong University, IIFSDU (no. 2014JC008).




